301. Among the following species, identify the isostructural pairs. $$N{F_3},NO_3^ - ,B{F_3},{H_3}{O^ + },H{N_3}$$
A
$$\left[ {N{F_3},NO_3^ - } \right]{\text{and}}\left[ {B{F_3},{H_3}{O^ + }} \right]$$
B
$$\left[ {N{F_3},H{N_3}} \right]{\text{and}}\left[ {NO_3^ - ,B{F_3}} \right]$$
C
$$\left[ {N{F_3},{H_3}{O^ + }} \right]{\text{and}}\left[ {NO_3^ - ,B{F_3}} \right]$$
D
$$\left[ {N{F_3},{H_3}{O^ + }} \right]{\text{and}}\left[ {N{H_3},B{F_3}} \right]$$
Answer :
$$\left[ {N{F_3},{H_3}{O^ + }} \right]{\text{and}}\left[ {NO_3^ - ,B{F_3}} \right]$$
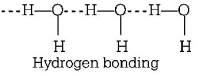
302. The high density of water compared to ice is due to
A
hydrogen bonding interactions
B
dipole-dipole interactions
C
dipole-induced dipole interactions
D
induced dipole-induced dipole interactions
Answer :
hydrogen bonding interactions
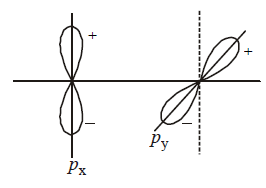
303. Which type of overlapping is shown by $$p\left( {{p_x},{p_y}\,{\text{and}}\,{p_z}} \right)$$ - orbitals ?
A
Two end to end and one sidewise overlap
B
Two sidewise and one end to end overlap
C
Three sidewise overlaps
D
Three end to end overlaps
Answer :
Two sidewise and one end to end overlap
304.
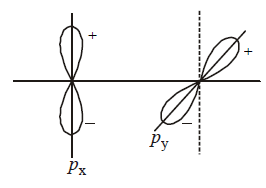
Main axis of a diatomic molecule is $$Z.\,AO's\,{p_x}$$ and $${p_y}$$ overlap to form which of the following orbitals?
A
$$\pi - MO$$
B
$$\sigma - MO$$
C
$$\delta - MO$$
D
$${\text{No bond will form}}$$
Answer :
$${\text{No bond will form}}$$
305. In which of the following species the interatomic bond angle is $${190^0}\,\,{28'}$$ ?
A
$$N{H_3},{\left( {B{F_4}} \right)^{ - 1}}$$
B
$${\left( {N{H_4}} \right)^ + },B{F_3}$$
C
$$N{H_3},B{F_4}$$
D
$${\left( {N{H_2}} \right)^{ - 1}},B{F_3}.$$
Answer :
$$N{H_3},{\left( {B{F_4}} \right)^{ - 1}}$$
306. The group having triangular planar structures is :
A
$$B{F_3},N{F_3},CO_3^{2 - }$$
B
$$CO_3^{2 - },NO_3^ - ,S{O_3}$$
C
$$N{H_3},S{O_3},CO_3^{2 - }$$
D
$$NC{l_3},BC{l_3},S{O_3}$$
Answer :
$$CO_3^{2 - },NO_3^ - ,S{O_3}$$
307. The molecule having one unpaired electron is :
A
$$NO$$
B
$$CO$$
C
$$C{N^ - }$$
D
$${O_2}$$
Answer :
$$NO$$
308.
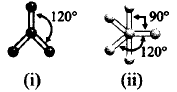
Which molecule is depicted by the given ball and stick models?
A
$$\left( {\text{i}} \right)BeC{l_2},\left( {{\text{ii}}} \right)C{H_4}$$
B
$$\left( {\text{i}} \right)B{F_3},\left( {{\text{ii}}} \right)PC{l_5}$$
C
$$\left( {\text{i}} \right)B{F_4},\left( {{\text{ii}}} \right)C{H_4}$$
D
$$\left( {\text{i}} \right)BeC{l_2},\left( {{\text{ii}}} \right)PC{l_5}$$
Answer :
$$\left( {\text{i}} \right)B{F_3},\left( {{\text{ii}}} \right)PC{l_5}$$
309.
Fill in the blanks with appropriate choice.
Bond order of $$N_2^ + $$ is $$\underline P $$ while that of $${N_2}$$ is $$\underline Q .$$ Bond order of $$O_2^ + $$ is $$\underline R $$ while that of $${O_2}$$ is $$\underline S .$$ $$N - N$$ bond distance $$\underline T ,$$ when $${N_2}$$ changes to $$N_2^ + $$ and when $${O_2}$$ changes to $$O_2^ + ,$$ the $$O - O$$ bond distance is $$\underline U .$$
$$P$$
$$Q$$
$$R$$
$$S$$
$$T$$
$$U$$
(a)
2
2.5
2.5
1
increases
decreases
(b)
2.5
3
2
1.5
decreases
increases
(c)
3
2
1.5
1
increases
decreases
(d)
2.5
3
2.5
2
increases
decreases
A
(a)
B
(b)
C
(c)
D
(d)
Answer :
(d)
310. Which of the following species exhibits the diamagnetic behaviour?
A
$$NO$$
B
$$O_2^{2 - }$$
C
$$O_2^ + $$
D
$${O_{2'}}$$
Answer :
$$O_2^{2 - }$$