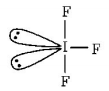
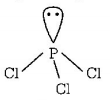
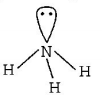
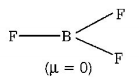
291. Which of the following molecules has trigonal planar geometry?
A
$$I{F_3}$$
B
$$PC{l_3}$$
C
$$N{H_3}$$
D
$$B{F_3}$$
Answer :
$$B{F_3}$$
292. A pair of electrons present between two identical non-metals
A
is shifted to one of the atoms
B
is shared equally between them
C
undergoes addition reactions
D
have same spin
Answer :
is shared equally between them
293. Which of the following pairs will have same bond order?
A
$${F_2}\,{\text{and}}\,O_2^{2 - }$$
B
$${N_2}\,{\text{and}}\,C{O_2}$$
C
$${O_2}\,{\text{and}}\,O_2^ - $$
D
$${N_2}\,{\text{and}}\,N_2^ + $$
Answer :
$${F_2}\,{\text{and}}\,O_2^{2 - }$$
294. In which of the following species the bond is non-directional
A
$$NC{l_3}$$
B
$$RbCl$$
C
$$BeC{l_2}$$
D
$$BC{l_3}$$
Answer :
$$RbCl$$
295. Which of the following species has unpaired electrons?
A
$${N_2}$$
B
$${F_2}$$
C
$$O_2^ - $$
D
$$O_2^{2 - }$$
Answer :
$$O_2^ - $$
296. In which of the following substances will hydrogen bond be strongest?
A
$$HCl$$
B
$${H_2}O$$
C
$$HI$$
D
$${H_2}S$$
Answer :
$${H_2}O$$
297. Molecular $$AB$$ has a bond length of $$1.61\mathop {\text{A}}\limits^{\text{o}} $$ and a dipole moment of $$0.38\,D.$$ The fractional charge on each atom ( absolute magnitude ) is : $$\left( {{e_0} = 4.802 \times {{10}^{ - 10}}esu} \right)$$
A
0.5
B
0.05
C
0
D
1.0
Answer :
0.05
298. In which one of the following molecules, the central atom said to adopt $$s{p^2}$$ hybridisation?
A
$$Be{F_2}$$
B
$$B{F_3}$$
C
$${C_2}{H_2}$$
D
$$N{H_3}$$
Answer :
$$B{F_3}$$
299. Four diatomic species are listed below in different sequences. Which of these presents the correct order of their increasing bond order?
A
$$O_2^ - < NO < C_2^{2 - } < He_2^ + $$
B
$$NO < C_2^{2 - } < O_2^ - < He_2^ + $$
C
$$C_2^{2 - } < He_2^ + < NO < O_2^ - $$
D
$$He_2^ + < O_2^ - < NO < C_2^{2 - }$$
Answer :
$$He_2^ + < O_2^ - < NO < C_2^{2 - }$$
300. Which of the following are arranged in the decreasing order of dipole moment?
A
$$C{H_3}Cl,C{H_3}Br,C{H_3}F$$
B
$$C{H_3}Cl,C{H_3}F,C{H_3}Br$$
C
$$C{H_3}Br,C{H_3}Cl,C{H_3}F$$
D
$$C{H_3}Br,C{H_3}F,C{H_3}Cl$$
Answer :
$$C{H_3}Cl,C{H_3}F,C{H_3}Br$$