161. The relationship between the dissociation energy of $${N_2}$$ and $$N_2^ + $$ is
A
dissociation energy of $$N_2^ + > \,$$ dissociation energy of $${N_2}$$
B
dissociation energy of $${N_2} = \,$$ dissociation energy of $$N_2^ + $$
C
issociation energy of $${N_2} > $$ dissociation energy of $$N_2^ + $$
D
dissociation energy of $${N_2}$$ can either be lower
or higher than the dissociation energy of $$N_2^ + $$
Answer :
issociation energy of $${N_2} > $$ dissociation energy of $$N_2^ + $$
162. The geometry of $${H_2}S$$ and its dipole moment are
A
angular and non-zero
B
angular and zero
C
linear and non-zero
D
linear and zero
Answer :
angular and non-zero
163. For two ionic solids $$CaO$$ and $$KI$$ , identify the wrong statement among the following.
A
Lattice energy of $$CaO$$ is much larger than that of $$KI$$
B
$$KI$$ is soluble in benzene
C
$$KI$$ has lower melting point
D
$$CaO$$ has higher melting point
Answer :
$$KI$$ is soluble in benzene
164. In a diatomic molecule the bond distance is $$1 \times {10^{ - 8}}\,cm.$$ Its dipole moment is $$1.2\,D.$$ What is the fractional electronic charge on each atom?
A
0.50
B
1.2 × 10-10
C
0.25
D
1.2
Answer :
0.25
165.
The most stable shape of $$Cl{F_3}$$ is shown by

A
only (i)
B
(i) and (iii)
C
only (ii)
D
only (iii)
Answer :
only (i)
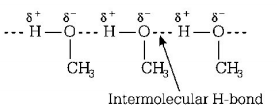
166. What is the dominant intermolecular force on bond that must be overcome in converting liquid $$C{H_3}OH$$ to a gas?
A
Hydrogen bonding
B
Dipole-dipole interaction
C
Covalent bonds
D
London or dispersion force
Answer :
Hydrogen bonding
167. Which of the following compounds shows maximum hydrogen bond strength?
A
$$HF$$
B
$${H_2}O$$
C
$$N{H_3}$$
D
$$C{H_3}OH$$
Answer :
$$HF$$
168.
In which pair of molecules is the permanent dipole in molecule I greater than that in molecule II ?
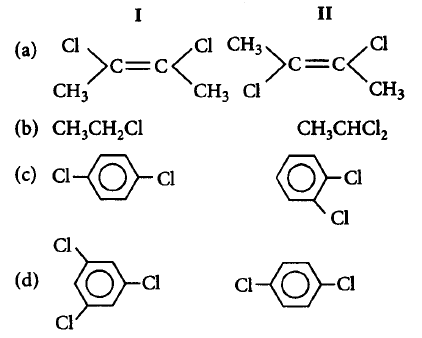
A
(a)
B
(b)
C
(c)
D
(d)
Answer :
(a)
169. Which one of the following pairs of molecules will have permanent dipole moments for both members?
A
$$N{O_2}\,\,{\text{and}}\,\,C{O_2}$$
B
$$N{O_2}\,\,{\text{and}}\,\,\,{O_3}$$
C
$$Si{F_4}\,\,\,{\text{and}}\,\,\,C{O_2}$$
D
$$Si{F_4}\,\,{\text{and}}\,\,N{O_2}$$
Answer :
$$N{O_2}\,\,{\text{and}}\,\,\,{O_3}$$
170.
Consider the molecules $$C{H_4},N{H_3}$$ and $${H_2}O.$$
Which of the given statements is false?
A
The $$H-O-H$$ bond angle in $${H_2}O$$ is larger than the $$H - C - H$$ bond angle in $$C{H_4}$$
B
The $$H - O - H$$ bond angle in $${H_2}O$$ is smaller than the $$H - N - H$$ bond angle in $$N{H_3}$$
C
The $$H - C - H$$ bond angle in $$C{H_4}$$ is larger than the $$H - N - H$$ bond angle in $$N{H_3}$$
D
The $$H - C - H$$ bond angle in $$C{H_4},$$ the $$H - N - H$$ bond angle in $$N{H_3}$$ and the $$H - O - H$$ bond angle in $${H_2}O$$ are all greater than $${90^ \circ }$$
Answer :
The $$H-O-H$$ bond angle in $${H_2}O$$ is larger than the $$H - C - H$$ bond angle in $$C{H_4}$$