151. Which one of the following species has plane triangular shape?
A
$${N_3}$$
B
$$NO_3^ - $$
C
$$NO_2^ - $$
D
$$C{O_2}$$
Answer :
$$NO_3^ - $$
152. Using $$MO$$ theory, predict which of the following species has the shortest bond length?
A
$$O_2^ + $$
B
$$O_2^ - $$
C
$$\,O_2^{2 - }$$
D
$$O_2^{2 + }$$
Answer :
$$O_2^{2 + }$$
153. Which of the following statements is not true regarding molecular orbital theory?
A
The atomic orbitals of comparable energies combine to form molecular orbitals.
B
An atomic orbital is monocentric while a molecular orbital is polycentric.
C
Bonding molecular orbital has higher energy than antibonding molecular orbital.
D
Molecular orbitals like atomic orbitals obey Aufbau principle for filling of electrons.
Answer :
Bonding molecular orbital has higher energy than antibonding molecular orbital.
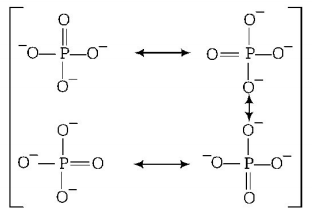
154. In $$PO_4^{3 - }$$ ion, the formal charge on each oxygen atom and $$P - O$$ bond order respectively are
A
$$- 0.75, 0.6$$
B
$$ 0.75, 1.0$$
C
$$- 0.75, 1.25$$
D
$$- 3, 1.25$$
Answer :
$$- 0.75, 1.25$$
155. Structure of $$Xe{F_4}$$ is
A
square planar
B
triangular
C
tetrahedral
D
octahedral
Answer :
square planar
156. Which of the following species contains equal number of $$\sigma $$ and $$\pi $$ -bonds?
A
$$HCO_3^ - $$
B
$$Xe{O_4}$$
C
$${\left( {CN} \right)_2}$$
D
$$C{H_2}{\left( {CN} \right)_2}$$
Answer :
$$Xe{O_4}$$
157. Considering the state of hybridisation of carbon atoms, find out the molecule among the following which is linear?
A
$$C{H_3} - C \equiv C - C{H_3}$$
B
$$C{H_2} = CH - C{H_2} - C \equiv CH$$
C
$$C{H_3} - C{H_2} - C{H_2} - C{H_3}$$
D
$$C{H_3} - C = CH - C{H_3}$$
Answer :
$$C{H_3} - C \equiv C - C{H_3}$$
158. Which of the following orbitals will not form sigma bond after overlapping?
A
$$s$$ - orbital and $$s$$ - orbital
B
$$s$$ - orbital and $${p_z}$$ - orbital
C
$${p_z}$$ - orbital and $${p_z}$$ - orbital
D
$${p_x}$$ - orbital and $${p_x}$$ - orbital
Answer :
$${p_x}$$ - orbital and $${p_x}$$ - orbital
159. The molecule which has pyramidal shape is :
A
$$PC{l_3}$$
B
$$S{O_3}$$
C
$$CO_3^{2 - }$$
D
$$NO_3^ - $$
Answer :
$$PC{l_3}$$
160. Which of the following compounds contain(s) no covalent bond(s)? \[\,KCl,P{H_3},{O_2},{B_2}{H_6},{H_2}S{O_4}\]
A
\[KCl,{B_2}{H_6},P{H_3}\]
B
\[KCl,{H_2}S{O_4}\,\]
C
\[KCl\]
D
\[KCl,{B_2}{H_6}\]
Answer :
\[KCl\]