281. A solution of sodium sulfate contains $$92 g$$ of $$N{a^ + }$$ ions per kilogram of water. The molality of $$N{a^ + }$$ ions in that solution in mol $$k{g^{ - 1}}$$ is :
A
12
B
4
C
8
D
16
Answer :
4
282. $$3\,moles$$ of $$P$$ and $$2\,moles$$ of $$Q$$ are mixed, what will be their total vapour pressure in the solution if their partial vapour pressures are $$80$$ and $$60\,torr$$ respectively ?
A
80$$\,torr$$
B
140$$\,torr$$
C
72$$\,torr$$
D
70$$\,torr$$
Answer :
72$$\,torr$$
283. Which of the aqueous equimolal solution will have its vapour pressure near to solvent ?
A
$${\text{Urea}}$$
B
$$Ba{\left( {N{O_3}} \right)_2}$$
C
$$NaN{O_3}$$
D
$$Al{\left( {N{O_3}} \right)_3}$$
Answer :
$${\text{Urea}}$$
284. Vapour pressure of chloroform $$\left( {CHC{l_3}} \right)$$ and dichloromethane $$\left( {C{H_2}C{l_2}} \right)$$ at $${25^ \circ }C$$ are $$200\,mm\,Hg$$ and $$41.5\,mm\,Hg$$ respectively. Vapour pressure of the solution obtained by mixing $$25.5\,g$$ of $$CHC{l_3}$$ and $$40\,g$$ of $$C{H_2}C{l_2}$$ at the same temperature will be : ( Molecular mass of $$CHC{l_3} = 119.5\,u$$ and molecular mass of $$C{H_2}C{l_2} = 85\,u$$ )
A
$$90.92\,mm\,Hg$$
B
$$115.0\,mm\,Hg$$
C
$$147.9\,mm\,Hg$$
D
$$285.5\,mm\,Hg$$
Answer :
$$90.92\,mm\,Hg$$
285. A solution of sucrose ( molar mass $$ = 342g\,mo{l^{ - 1}}$$ ) has been prepared by dissolving $$68.5\,g$$ of sucrose in $$1000\,g$$ of water. The freezing point of the solution obtained will be $$\left( {{k_f}\,{\text{for water}} = 1.86\,K\,kg\,mo{l^{ - 1}}} \right)$$
A
$$ - {0.372^ \circ }C$$
B
$$ - {0.520^ \circ }C$$
C
$$ + {0.372^ \circ }C$$
D
$$ - {0.570^ \circ }C$$
Answer :
$$ - {0.372^ \circ }C$$
286. Which of the following will have same value of van't Hoff factor as that of $${K_4}\left[ {Fe{{\left( {CN} \right)}_6}} \right]?$$
A
$$A{l_2}{\left( {S{O_4}} \right)_3}$$
B
$$AlC{l_3}$$
C
$$Al{\left( {N{O_3}} \right)_3}$$
D
$$Al{\left( {OH} \right)_3}$$
Answer :
$$A{l_2}{\left( {S{O_4}} \right)_3}$$
287.
Consider the two figures given below.
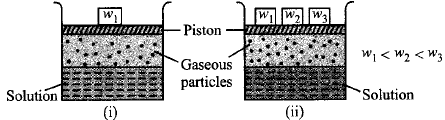
Which of the following statements regarding the experiment is true ?
A
The solubility of a gas in liquid in beaker (i) is greater than that in beaker (ii),
B
The solubility of a gas in beaker (i) is less than that in beaker (ii).
C
The solubility of a gas is equal in both beakers.
D
The solubility of a gas remains unaffected by change in weights.
Answer :
The solubility of a gas in beaker (i) is less than that in beaker (ii).
288. In mixture $$A$$ and $$B$$ components show -ve deviation as
A
$$\Delta {V_{mix}} > 0$$
B
$$\Delta {H_{mix}} < 0$$
C
$$A - B$$ interaction is weaker than $$A - A$$ and $$B - B$$ interaction
D
$$A - B$$ interaction is stronger than $$A - A$$ and $$B - B$$ interaction.
Answer :
$$A - B$$ interaction is stronger than $$A - A$$ and $$B - B$$ interaction.
289.
In three beakers labelled as $$(A), (B)$$ and $$(C),$$ $$100\,mL$$ of water, $$100\,mL$$ of $$1\,M$$ solution of glucose in water and $$100\,mL$$ of $$0.5\,M$$ solution of glucose in water are taken respectively and kept at same temperature.
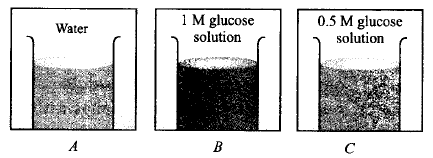
Which of the following statements is correct ?
A
Vapour pressure in all the three beakers is same.
B
Vapour pressure of beaker $$B$$ is highest.
C
Vapour pressure of beaker $$C$$ is highest.
D
Vapour pressure of beaker $$B$$ is lower than that of $$C$$ and vapour pressure of beaker $$C$$ is lower than that of $$A.$$
Answer :
Vapour pressure of beaker $$B$$ is lower than that of $$C$$ and vapour pressure of beaker $$C$$ is lower than that of $$A.$$
290. The vapour pressure, at a given temperature, of an ideal solution containing $$0.2$$ $$mole$$ of a non-volatile solute and $$0.8$$ $$mole$$ of solvent is $$60$$ $$mm$$ of $$Hg.$$ The vapour pressure of the pure solvent at the same temperature is
A
$$150\,mm\,{\text{of}}\,Hg$$
B
$$60\,mm\,{\text{of}}\,Hg$$
C
$$75\,mm\,{\text{of}}\,Hg$$
D
$$120\,mm\,{\text{of}}\,Hg$$
Answer :
$$75\,mm\,{\text{of}}\,Hg$$