211.
On mixing, heptane and octane form an ideal solution. At $$373 K,$$ the vapour pressures of the two liquid components (heptane and octane) are $$105$$ $$kPa$$ and $$45$$ $$kPa$$ respectively. Vapour pressure of the solution obtained by mixing $$25.0 g$$
of heptane and $$435 g$$ of octane will be
( molar mass of heptane = $${100\,g\,mo{l^{ - 1}}}$$ and of octane = $${114g\,mo{l^{ - 1}}}$$ )
A
72.0 $$kPa$$
B
36.1$$ $$kPa$$
C
96.2 $$kPa$$
D
144.5 $$kPa$$
Answer :
72.0 $$kPa$$
212. At $${20^ \circ }C$$ and $$1.00\,atm$$ partial pressure of $${H_2},18\,mL$$ of $${H_2}\left( {STP} \right)$$ dissolves in $$1\,L$$ of water. If $$2\,L$$ of water is exposed to a gaseous mixture having a total pressure of $$1425\,torr$$ ( excluding the vapour pressure of water ) and containing $$80\% \,{H_2}$$ by volume, the volume of $${H_2}\left( {STP} \right)$$ dissolved is
A
27$$\,mL$$
B
54$$\,mL$$
C
33.75$$\,mL$$
D
67.50$$\,mL$$
Answer :
54$$\,mL$$
213. If $$\alpha $$ is the degree of dissociation of $$N{a_2}S{O_4},$$ the van't Hoff's factor (i) used for calculating the molecular mass is
A
$$1 + \alpha $$
B
$$1 - \alpha $$
C
$$1 + 2\alpha $$
D
$$1 - 2\alpha $$
Answer :
$$1 + 2\alpha $$
214. What will be the freezing point of a $$0.5\,m\,KCl$$ solution ? The molal freezing point constant of water is $${1.86^ \circ }C\,{m^{ - 1}}.$$
A
$$ - {1.86^ \circ }C$$
B
$$ - {0.372^ \circ }C$$
C
$$ - {3.2^ \circ }C$$
D
$${0^ \circ }C$$
Answer :
$$ - {1.86^ \circ }C$$
215. Which of the following $$0.10$$ $$M$$ aqueous solution will have the lowest freezing point?
A
$$A{l_2}{\left( {S{O_4}} \right)_3}$$
B
$${C_5}{H_{10}}{O_5}$$
C
$$Kl$$
D
$${C_{12}}{H_{22}}{O_{11}}$$
Answer :
$$A{l_2}{\left( {S{O_4}} \right)_3}$$
216. The solution containing $$4.0\,g$$ of a polyvinyl chloride polymer in 1 litre of dioxane was found to have an osmotic pressure $$6.0 \times {10^{ - 4}}$$ atmosphere at $$300\,K,$$ the value of $$R$$ used is 0.082 litre atmosphere $$mol{e^{ - 1}}{K^{ - 1}}.$$ The molecular mass of the polymer was found to be
A
$$3.0 \times {10^2}$$
B
$$1.6 \times {10^5}$$
C
$$5.6 \times {10^4}$$
D
$$6.4 \times {10^2}$$
Answer :
$$1.6 \times {10^5}$$
217. A binary liquid solution is prepared by mixing $$n-$$heptane and ethanol. Which one of the following statements is correct regarding the behaviour of the solution?
A
The solution is non-ideal, showing $$- ve$$ deviation from
Raoult’s Law.
B
The solution is non-ideal, showing $$+ ve$$ deviation from
Raoult’s Law.
C
$$n-$$heptane shows $$+ ve$$ deviation while ethanol shows
-ve deviation from Raoult’s Law.
D
The solution formed is an ideal solution
Answer :
The solution is non-ideal, showing $$+ ve$$ deviation from
Raoult’s Law.
218. A mixture of ethyl alcohol and propyl alcohol has a vapour pressure of $$290\, mm$$ at $$300\,K.$$ The vapour pressure of propyl alcohol is $$200 \,mm.$$ If the mole fraction of ethyl alcohol is $$0.6,$$ its vapour pressure $$\left( {{\text{in}}\,\,mm} \right)$$ at the same temperature will be
A
360
B
350
C
300
D
700
Answer :
350
219. At 80° C, the vapour pressure of pure liquid $$'A’$$ is $$520 mm$$ $$Hg$$ and that of pure liquid $$'B’$$ is $$1000 mm$$ $$Hg.$$ If a mixture solution of $$'A’$$ and $$'B’$$ boils at $${80^ \circ }C$$ and 1 atm pressure, the amount of $$'A’$$ in the mixture is $$\left( {1\,atm\, = 760\,mm\,Hg} \right)$$
A
52 mol percent
B
34 mol percent
C
48 mol percent
D
50 mol percent
Answer :
50 mol percent
220.
Grapes placed in three beakers $$X, Y$$ and $$Z$$ containing different types of solutions are shown in figures.
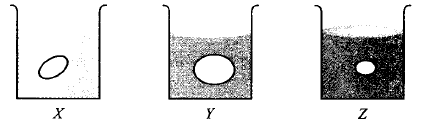
If beaker $$X$$ contains water, $$Y$$ and $$Z$$ contain
A
$$Y$$ - hypotonic solution, $$Z$$ - hypertonic solution
B
$$Y$$ - hypertonic solution, $$Z$$ - hypotonic solution
C
$$Y$$ and $$Z$$ - isotonic solutions
D
$$Y$$ and $$Z$$ - hypotonic solutions
Answer :
$$Y$$ - hypotonic solution, $$Z$$ - hypertonic solution