241. A solution containing components $$A$$ and $$B$$ follows Raoult’s law, when
A
$$A- B$$ attraction force is greater than $$A- A$$ and $$B-B$$
B
$$A-B$$ attraction force is less than $$A - A$$ and $$B-B$$
C
$$A -B$$ attraction force remains same as $$A - A$$ and $$B-B$$
D
volurne of solution is different from sum of volumes of solute and solvent
Answer :
$$A -B$$ attraction force remains same as $$A - A$$ and $$B-B$$
242. $$250\,mL$$ of sodium carbonate solution contains $$2.65\,g$$ of $$N{a_2}C{O_3}.$$ If $$10\,mL$$ of this solution is diluted to $$500\,mL,$$ the concentration of the diluted acid will be
A
0.01$$\,M$$
B
0.001$$\,M$$
C
0.05$$\,M$$
D
0.002$$\,M$$
Answer :
0.002$$\,M$$
243. $$12\,g$$ glucose $$\left( {{C_6}{H_{12}}{O_6}} \right)$$ is added to $$178.2 g$$ water. The vapour pressure of water (in torr) for this aqueous solution is :
A
752.4
B
759.0
C
7.6
D
76.0
Answer :
752.4
244. A $$0.001\,molal$$ solution of $$\left[ {Pt{{\left( {N{H_3}} \right)}_4}C{l_4}} \right]$$ in water had a freezing point depression of $${0.0054^ \circ }C.$$ If $${K_f}$$ for water is $$1.80,$$ the correct formulation for the above molecule is
A
$$\left[ {Pt{{\left( {N{H_3}} \right)}_4}C{l_3}} \right]Cl$$
B
$$\left[ {Pt{{\left( {N{H_3}} \right)}_4}C{l_2}} \right]C{l_2}$$
C
$$\left[ {Pt{{\left( {N{H_3}} \right)}_4}Cl} \right]C{l_3}$$
D
$$\left[ {Pt{{\left( {N{H_3}} \right)}_4}C{l_4}} \right]$$
Answer :
$$\left[ {Pt{{\left( {N{H_3}} \right)}_4}C{l_2}} \right]C{l_2}$$
245.
Pure water freezes at $$273 K$$ and 1 bar. The addition of $$34.5 g$$ of ethanol to $$500 g$$ of water changes the freezing point of
the solution. Use the freezing point depression constant of water as $$2\,K\,kg\,mo{l^{ - 1}}.$$ The figures shown below represent
plots of vapour pressure $$(V.P.)$$ versus temperature $$(T).$$ [ molecular weight of ethanol is $$46\,g\,mo{l^{ - 1}}$$ ] Among the
following, the option representing change in the freezing point is
A
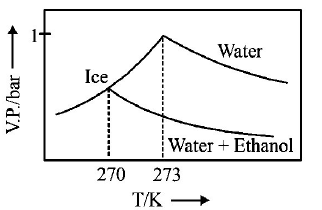

B
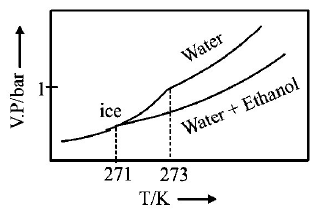

C
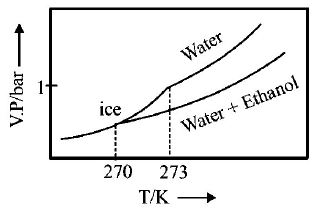

D
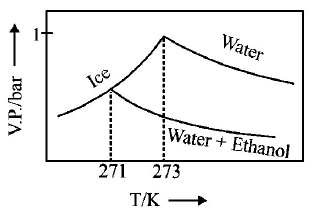

Answer :


246. Why is the molecular mass determined by measuring colligative property in case of some solutes is abnormal ?
A
Due to association or dissociation of solute molecules.
B
Due to insolubility of solute molecules.
C
Due to decomposition of solute molecules.
D
Due to large size of solute molecules
Answer :
Due to association or dissociation of solute molecules.
247. The vapour pressure of two liquids $$P$$ and $$Q$$ are $$80$$ and $$60$$ $$torr,$$ respectively. The total vapour pressure of solution obtained by mixing $$3$$ $$moles$$ of $$P$$ and $$2$$ $$moles$$ of $$Q$$ would be
A
$$140\,torr$$
B
$$20\,torr$$
C
$$68\,torr$$
D
$$72\,torr$$
Answer :
$$72\,torr$$
248. In amalgam of mercury with sodium, solvent is
A
mercury
B
sodium
C
amalgam
D
none of these.
Answer :
sodium
249. On dissolving sugar in water at room temperature solution feels cool to touch. Under which of the following cases dissolution of sugar will be most rapid ?
A
Sugar crystals in cold water
B
Sugar crystals in hot water
C
Powdered sugar in cold water
D
Powdered sugar in hot water
Answer :
Powdered sugar in hot water
250. People taking lot of salt experience puffiness or swelling of the body due to
A
water retention in tissue cells and intercellular spaces because of osmosis
B
water loss from the cells through skin tissues
C
capillary action of water through skin pores
D
excessive thirst and drinking more water
Answer :
water retention in tissue cells and intercellular spaces because of osmosis