41. The enthalpy change for a reaction does not depend upon
A
use of different reactants for the same product
B
the nature of intermediate reaction steps
C
the differences in initial or final temperatures of involved substances
D
the physical states of reactants and products
Answer :
the nature of intermediate reaction steps
42. Heat of neutralization of a strong acid $$HA$$ and a weaker acid $$HB$$ with $$KOH$$ are $$ - 13.7$$ and $$ - 12.7\,k\,cal\,mo{l^{ - 1}}.$$ When $$1\,mole$$ of $$KOH$$ was added to a mixture containing $$1\,mole$$ each of $$HA$$ and $$HB,$$ the heat change was $$ - 13.5\,kcal.$$ In what ratio is the base distributed between $$HA$$ and $$HB.$$
A
3 : 1
B
1 : 3
C
4 : 1
D
1 : 4
Answer :
4 : 1
43. If the heat change at constant volume for decomposition of silver oxide is $$80.25\,kJ,$$ what will be the heat change at constant pressure ?
A
$$80.25\,kJ$$
B
$$ > 80.25\,kJ$$
C
$$ < 80.25\,kJ$$
D
$$160.50\,kJ$$
Answer :
$$ > 80.25\,kJ$$
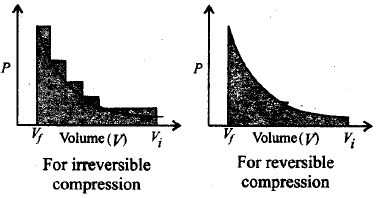
44. The pressure-volume work for an ideal gas can be calculated by using the expression $$W = - \int\limits_{{V_t}}^{{V_f}} {{P_{ex}}\,dV.} $$ The work can also be calculated from the $$PV - $$ plot by using the area under the curve within the specified limits. When an ideal gas is compressed (i) reversibly or (ii) irreversibly from volume $${V_i}$$ to $${V_f}.$$ Choose the correct option.
A
$${W_{{\text{reversible}}}} = {W_{{\text{irreversible}}}}$$
B
$${W_{{\text{reversible}}}} < {W_{{\text{irreversible}}}}$$
C
$${W_{{\text{reversible}}}} > {W_{{\text{irreversible}}}}$$
D
$${W_{{\text{reversible}}}} = {W_{{\text{irreversible}}}} + {P_{ex}}\,.\Delta V$$
Answer :
$${W_{{\text{reversible}}}} < {W_{{\text{irreversible}}}}$$
45. Dissolution of ammonium chloride in water is an endothermic reaction, yet it is a spontaneous process. This is due to the fact that
A
$$\Delta H\,\,{\text{is}}\,\, + ve,\Delta S\,\,{\text{is}}\,\, - ve$$
B
$$\Delta H\,\,{\text{is}}\,\, - ve,\Delta S\,\,{\text{is}}\,\, + ve$$
C
$$\Delta H\,\,{\text{is}}\,\, + ve,\Delta S\,\,{\text{is}}\,\, + ve\,\,$$ $${\text{and}}\,\,\Delta H < T\Delta S$$
D
$$\Delta H\,\,{\text{is}}\,\, + ve\,\,{\text{and}}\,\,\Delta H > T\Delta S$$
Answer :
$$\Delta H\,\,{\text{is}}\,\, + ve,\Delta S\,\,{\text{is}}\,\, + ve\,\,$$ $${\text{and}}\,\,\Delta H < T\Delta S$$
46.
For a reaction : $$X \to Y + Z$$
Absolute entropies are $$X = 120\,J\,{K^{ - 1}}\,mo{l^{ - 1}},$$ $$Y = 213.8\,J\,{K^{ - 1}}\,mo{l^{ - 1}}$$ and $$Z = 197.9\,J\,{K^{ - 1}}\,mo{l^{ - 1}}.$$
What will be the entropy change at $$298\,K$$ and $$1\,atm?$$
A
$$291.7\,J\,{K^{ - 1}}$$
B
$$255\,J\,{K^{ - 1}}$$
C
$$213.8\,J\,{K^{ - 1}}$$
D
$$257.3\,J\,{K^{ - 1}}$$
Answer :
$$291.7\,J\,{K^{ - 1}}$$
47. The value for $$\Delta U$$ for the reversible isothermal evaporation of $$90\,g$$ water at $$100{\,^ \circ }C$$ will be $$\left( {\Delta {H_{{\text{evap}}}}} \right.$$ of water $$ = 40.8\,kJ\,mo{l^{ - 1}},$$ $$\left. {R = 8.314\,J\,{K^{ - 1}}\,mo{l^{ - 1}}} \right)$$
A
$$4800\,kJ$$
B
$$188.494\,kJ$$
C
$$40.8\,kJ$$
D
$$125.03\,kJ$$
Answer :
$$188.494\,kJ$$
48.
At what temperature liquid water will be in equilibrium with water vapour ?
$$\Delta {H_{{\text{vap}}}} = 40.73\,kJ\,mo{l^{ - 1}},$$ $$\Delta {S_{{\text{vap}}}} = 0.109\,kJ\,{K^{ - 1}}\,mo{l^{ - 1}}$$
A
282.4$$\,K$$
B
373.6$$\,K$$
C
100$$\,K$$
D
400$$\,K$$
Answer :
373.6$$\,K$$
49. For which of the process, $$\Delta S$$ is negative ?
A
$${H_{2\left( g \right)}} \to 2{H_{\left( g \right)}}$$
B
$${N_{2\left( {g,1\,atm} \right)}} \to {N_{2\left( {g,8\,atm} \right)}}$$
C
$$2S{O_{3\left( g \right)}} \to 2S{O_{2\left( g \right)}} + {O_{2\left( g \right)}}$$
D
$${C_{{\text{(diamond)}}}} \to {C_{{\text{(graphite)}}}}$$
Answer :
$${N_{2\left( {g,1\,atm} \right)}} \to {N_{2\left( {g,8\,atm} \right)}}$$
50.
For the reactions,
$$\eqalign{
& 2C + {O_2} \to 2\,C{O_2},\,\,\,\,\,\,\,\,\,\Delta H = - 393\,J \cr
& 2Zn + {O_2} \to 2ZnO\,;\,\,\,\,\,\,\Delta H = - 412\,J \cr} $$
A
carbon can oxidise $$Zn$$
B
oxidation of carbon is not feasible
C
oxidation of $$Zn$$ is not feasible
D
$$Zn$$ can oxidise carbon.
Answer :
$$Zn$$ can oxidise carbon.