21. The decomposition of a substance follows first order kinetics. If its concentration is reduced to $$\frac{1}{8}$$ of its initial value in 12 minutes, the rate constant of the decomposition system is
A
\[\left( \frac{2.303}{12}\log \frac{1}{8} \right){{\min }^{-1}}\]
B
\[\left( \frac{2.303}{12}\log 8 \right){{\min }^{-1}}\]
C
\[\left( \frac{0.693}{12} \right){{\min }^{-1}}\]
D
\[\left( \frac{1}{12}\log 8 \right){{\min }^{-1}}\]
Answer :
\[\left( \frac{2.303}{12}\log 8 \right){{\min }^{-1}}\]
22. For the reaction, $$4N{H_3} + 5{O_2} \to 4NO + 6{H_2}O,$$ if the rate of disappearance of $$N{H_3}$$ is $$3.6 \times {10^{ - 3}}\,mol\,{L^{ - 1}}\,{s^{ - 1}},$$ what is the rate of formation of $${H_2}O?$$
A
$$5.4 \times {10^{ - 3}}mol\,{L^{ - 1}}\,{s^{ - 1}}$$
B
$$3.6 \times {10^{ - 3}}\,mol\,{L^{ - 1}}\,{s^{ - 1}}$$
C
$$4 \times {10^{ - 4}}mol\,{L^{ - 1}}{s^{ - 1}}$$
D
$$0.6 \times {10^{ - 4}}\,mol\,{L^{ - 1}}\,{s^{ - 1}}$$
Answer :
$$5.4 \times {10^{ - 3}}mol\,{L^{ - 1}}\,{s^{ - 1}}$$
23.
For the non - stoichimetre reaction $$2A + B → C+D,$$ the following kinetic data were obtained in three separate experiments, all at $$298 K.$$
Initial Concentration $$\left( A \right)$$
Initial Concentration $$\left( B \right)$$
Initial rate of formation of $$C\left( {mol\,{L^{ -
1}}\,{s^{ - 1}}} \right)$$
$$0.1\,M$$
$$0.1\,M$$
$$1.2 \times {10^{ - 3}}$$
$$0.1\,M$$
$$0.2\,M$$
$$1.2 \times {10^{ - 3}}$$
$$0.2\,M$$
$$0.1\,M$$
$$2.4 \times {10^{ - 3}}$$
The rate law for the formation of $$C$$ is :
A
$$\frac{{dc}}{{dt}} = k\left[ A \right]\left[ B \right]$$
B
$$\frac{{dc}}{{dt}} = k{\left[ A \right]^2}\left[ B \right]$$
C
$$\frac{{dc}}{{dt}} = k\left[ A \right]{\left[ B \right]^2}$$
D
$$\frac{{dc}}{{dt}} = k\left[ A \right]$$
Answer :
$$\frac{{dc}}{{dt}} = k\left[ A \right]$$
24. A first order reaction is $$20\% $$ complete in 10 minutes. What is the specific rate constant for the reaction?
A
\[\text{0}\text{.0970}\,\text{mi}{{\text{n}}^{-1}}\]
B
\[\text{0}\text{.009}\,{{\min }^{-1}}\]
C
\[\text{0}\text{.0223}\,\text{mi}{{\text{n}}^{-1}}\]
D
\[\text{2}\text{.223}\,\text{mi}{{\text{n}}^{-1}}\]
Answer :
\[\text{0}\text{.0223}\,\text{mi}{{\text{n}}^{-1}}\]
25.
A schematic plot of In $${K_{ep}}$$ versus inverse of temperature for a reaction is shown below
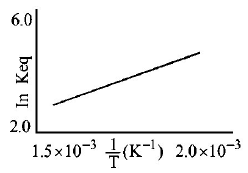
The reaction must be
A
highly spontaneous at ordinary temperature
B
one with negligible enthalpy change
C
endothermic
D
exothermic
Answer :
exothermic
26. The rate constant of a reaction is \[0.0693\,{{\min }^{-1}}.\] Starting with $$10\,mol,$$ the rate of the reaction after $$10\,\min $$ is
A
\[0.0693\,mol\,{{\min }^{-1}}\]
B
\[0.0693\times 2\,mol\,{{\min }^{-1}}\]
C
\[0.0693\times 5\,mol\,{{\min }^{-1}}\]
D
\[0.0693\times {{\left( 5 \right)}^{2}}mol\,{{\min }^{-1}}\]
Answer :
\[0.0693\times 5\,mol\,{{\min }^{-1}}\]
27. Consider a reaction $$aG + bH →$$ Products. When concentration of both the reactants $$G$$ and $$H$$ is doubled, the rate increases by eight times. However, when concentration of $$G$$ is doubled keeping the concentration of $$H$$ fixed, the rate is doubled. The overall order of the reaction is
A
0
B
1
C
2
D
3
Answer :
3
28. For a certain reaction, rate $$ = k \times {\left[ {{H^ + }} \right]^n}.$$ If $$pH$$ of reaction changes from two to one, the rate becomes 100 times of its value at $$pH = 2,$$ the order of reaction is –
A
1
B
2
C
0
D
3
Answer :
2
29.
For a reaction $$A \to $$ Products, a plot of $${\text{log}}\,{t_{\frac{1}{2}}}$$ versus $${\text{log}}\,{a_0}$$ is shown in the figure. If the initial concentration of $$A$$ is represented by $${a_0},$$ the order of the reaction is

A
one
B
zero
C
two
D
three
Answer :
zero
30. A radioactive element gets spilled over the floor of a room. Its half-life period is 30 days. If the initial velocity is ten times the permissible value, after how many days will it be safe to enter the room?
A
100 days
B
1000 days
C
300 days
D
10 days.
Answer :
100 days