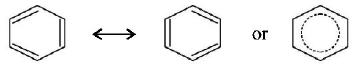
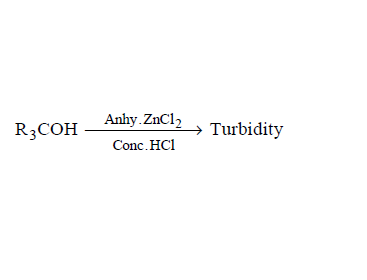
321. The bond order of individual carbon-carbon bonds in benzene is
A
one
B
two
C
between one and two
D
one and two alternately
Answer :
between one and two
322. The principle involved in paper chromatography is
A
adsorption
B
partition
C
solubility
D
volatility
Answer :
partition
323. Which one of the following orders is correct regarding the $$-I$$ -effect of the substituents ?
A
$$ - N{R_2} < - OR > - F$$
B
$$ - N{R_2} > - OR > - F$$
C
$$ - N{R_2} < - OR < - F$$
D
$$ - N{R_2} > - OR < - F$$
Answer :
$$ - N{R_2} < - OR < - F$$
325. With respect to the conformers of ethane, which of the following statements is true?
A
Bond angle remains same but bond length changes
B
Bond angle changes but bond length remains same
C
Both bond angle and bond length change
D
Both bond angles and bond length remain same
Answer :
Both bond angles and bond length remain same
326. Which of the following statements is not correct ?
A
Carbocation posses sextet of electrons.
B
The order of carbocation stability is :
$$\mathop C\limits^ + {H_3} > {\left( {C{H_3}} \right)_2}\mathop C\limits^ + H > {\left( {C{H_3}} \right)_3}\mathop C\limits^ + $$
$$\mathop C\limits^ + {H_3} > {\left( {C{H_3}} \right)_2}\mathop C\limits^ + H > {\left( {C{H_3}} \right)_3}\mathop C\limits^ + $$
C
Carbocations have trigonal planar shape
D
Carbocations are formed by heterolytic cleavage
Answer :
The order of carbocation stability is :
$$\mathop C\limits^ + {H_3} > {\left( {C{H_3}} \right)_2}\mathop C\limits^ + H > {\left( {C{H_3}} \right)_3}\mathop C\limits^ + $$
$$\mathop C\limits^ + {H_3} > {\left( {C{H_3}} \right)_2}\mathop C\limits^ + H > {\left( {C{H_3}} \right)_3}\mathop C\limits^ + $$
327. Which one of the following pairs represents stereoisomerism?
A
Chain isomerism and rotational isomerism
B
Structural isomerism and geometrical isomerism
C
Linkage isomerism and geometrical isomerism
D
Optical isomerism and geometrical isomerism
Answer :
Optical isomerism and geometrical isomerism
328. In an $${S_N}1$$ reaction on chiral centres there is
A
100% racemisation
B
inversion more than retention leading to partial racemisation
C
100% retention
D
100% inversion
Answer :
inversion more than retention leading to partial racemisation
329. Which one of the following does not have $$s{p^2}$$ hybridized carbon ?
A
Acetonitrile
B
Acetic acid
C
Acetone
D
Acetamide
Answer :
Acetonitrile