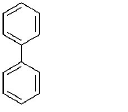
281. Maximum $$-I$$ effect is exerted by the group
A
$$ - {C_6}{H_5}$$
B
$$ - OC{H_3}$$
C
$$ - Cl$$
D
$$ - N{O_2}$$
Answer :
$$ - N{O_2}$$
282. Which of the following organic compounds has same hybridisation as its combustion $$\left( {C{O_2}} \right)$$ product?
A
Ethane
B
Ethyne
C
Ethene
D
Ethanol
Answer :
Ethyne
283.
Match the entries of List I with appropriate entries of List II and select the correct answer using the codes given below the lists :
List I (Aromatic compound)
List II (Factor responsible for electrophilic substitution)
P.
$${C_6}{H_5}CH = C{H_2}$$
1.
$$ + E$$ effect
Q.
$${C_6}{H_5}CC{l_3}$$
2.
$$ + M$$ effect
R.
$${C_6}{H_5}OCOC{H_3}$$
3.
Hyperconjugation
S.
$${C_6}{H_5}OH$$ in presence of $$NaOH$$
4.
$$ - I$$ effect
A
P - 1; Q - 2, 3; R - 3; S - 4
B
P - 2; Q - 3, 4; R - 1, 2; S - 1, 2
C
P - 1; Q - 3, 4; R - 3, 4; S - 1, 2
D
P - 2; Q - 1, 2; R - 1, 2; S - 3, 4
Answer :
P - 2; Q - 3, 4; R - 1, 2; S - 1, 2
284. Distillation under reduced pressure is generally used to purify those liquids which
A
have very low boiling points
B
are volatile
C
have high boiling points and which decompose below their boiling points
D
have a large difference in their boiling points
Answer :
have high boiling points and which decompose below their boiling points
285.
Rate of the reaction
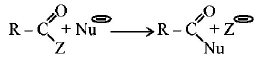
is fastest when $$Z$$ is
A
$$O{C_2}{H_5}$$
B
$$N{H_2}$$
C
$$Cl$$
D
$$OCOC{H_3}$$
Answer :
$$Cl$$
288. Due to the presence of an unpaired electron, free radicals are :
A
cations
B
anions
C
chemically inactive
D
chemically reactive
Answer :
chemically reactive
289.
Match the column I with column II and mark the appropriate choice.
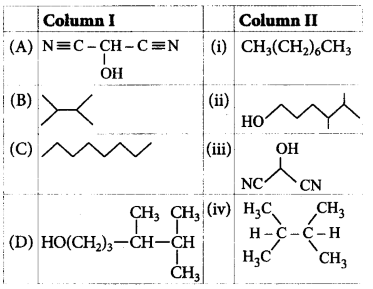
A
A - iii, B - iv, C - i, D - ii
B
A - iv, B - iii, C - ii, D - i
C
A - i, B - ii, C - iv, D - iii
D
A - ii, B - iii, C - i, D - iv
Answer :
A - iii, B - iv, C - i, D - ii
290. Among the following, the molecule with the highest dipole moment is :
A
$$C{H_3}Cl$$
B
$$C{H_2}C{l_2}$$
C
$$CHC{l_3}$$
D
$$CC{l_4}$$
Answer :
$$C{H_3}Cl$$