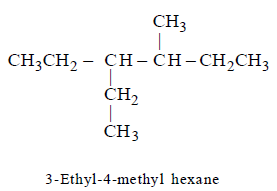251. Which of the following resonating structures of 1- methoxy - 1, 3 - butadiene is least stable?
A
$$\mathop {\text{C}}\limits^\Theta {H_2} - CH = CH - CH = \mathop O\limits^ \oplus - C{H_3}$$
B
$$C{H_2} = CH - \mathop {\text{C}}\limits^\Theta H - CH = \mathop O\limits^ \oplus - C{H_3}$$
C
$$\mathop {\text{C}}\limits^\Theta {H_2} - C\mathop H\limits^ \oplus - CH = CH - O - C{H_3}$$
D
$$C{H_2} = CH - \mathop {\text{C}}\limits^\Theta H - \mathop C\limits^ \oplus H - O - C{H_3}$$
Answer :
$$\mathop {\text{C}}\limits^\Theta {H_2} - C\mathop H\limits^ \oplus - CH = CH - O - C{H_3}$$
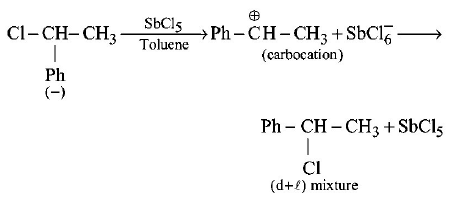
252. A solution of $$( - ) - 1 -$$ chloro $$- 1 -$$ phenylethane in toluene racemises slowly in the presence of a small amount of $$SbC{l_5},$$ due to the formation of :
A
carbanion
B
Carbene
C
carbocation
D
free radical
Answer :
carbocation
253. An organic compound contains $$69\% $$ carbon and $$4.8\% $$ hydrogen, the remainder being oxygen. What will be the masses of carbon dioxide and water produced when $$0.20\,g$$ of this substance is subjected to complete combustion?
A
$$0.69\,g\,\,{\text{and}}\,\,0.048\,g$$
B
$$0.506\,g\,\,{\text{and}}\,\,0.086\,g$$
C
$$0.345\,g\,\,\,{\text{and}}\,\,0.024\,g$$
D
$$0.91\,g\,\,\,{\text{and}}\,\,\,\,0.72\,g$$
Answer :
$$0.506\,g\,\,{\text{and}}\,\,0.086\,g$$
254.
The stability of carbanions in the following compounds,
$$\left( {\text{i}} \right)RCH = \bar CH$$
$$\left( {{\text{ii}}} \right)$$ 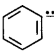
$$\left( {{\text{iii}}} \right){R_2}C = \bar CH$$
$$\left( {{\text{iv}}} \right){R_3}C - \bar C{H_2}$$
is in the order of
A
(iv) > (ii) > (iii) > (i)
B
(i) > (iii) > (ii) > (iv)
C
(i) > (ii) > (iii) > (iv)
D
(ii) > (iii) > (iv) > (i)
Answer :
(i) > (ii) > (iii) > (iv)
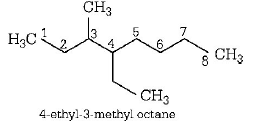
255.
Name of the compound given below
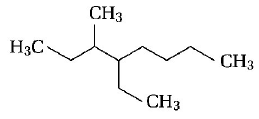
A
2, 3-diethylheptane
B
5-ethyl-6-methyloctane
C
4-ethyl-3-methyloctane
D
3-methyl-4-ethyloctane
Answer :
4-ethyl-3-methyloctane
256. The correct order regarding the electronegativity of hybrid orbitals of carbon is
A
$$sp > s{p^2} < s{p^3}$$
B
$$sp > s{p^2} > s{p^3}$$
C
$$sp < s{p^2} > s{p^3}$$
D
$$sp < s{p^2} < s{p^3}$$
Answer :
$$sp > s{p^2} > s{p^3}$$
257. Which of the following compounds exhibits stereoisomerism?
A
2 - methylbutene - 1
B
3 - methylbutyne - 1
C
3 - methylbutanoicacid
D
2 - methylbutanoic acid
Answer :
2 - methylbutanoic acid
258.
The correct IUPAC name of the following compound 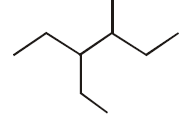 is :
is :
A
4 - methyl - 3 - ethylhexane
B
3 - ethyl - 4 - methylhexane
C
3, 4 - ethylmethylhexane
D
4 - ethyl - 3 - methylhexane
Answer :
3 - ethyl - 4 - methylhexane
259. The increasing order of stability of the following free radicals is
A
$${\left( {{C_6}{H_5}} \right)_2}\mathop C\limits^ \bullet H < {\left( {{C_6}{H_5}} \right)_3}\mathop C\limits^ \bullet < {\left( {C{H_3}} \right)_3}\mathop C\limits^ \bullet < {\left( {C{H_3}} \right)_2}\mathop C\limits^ \bullet H$$
B
$${\left( {C{H_3}} \right)_2}\mathop C\limits^ \bullet H < {\left( {C{H_3}} \right)_3}\mathop C\limits^ \bullet < {\left( {{C_6}{H_5}} \right)_2}\mathop C\limits^ \bullet H < {\left( {{C_6}{H_5}} \right)_3}\mathop C\limits^ \bullet $$
C
$${\left( {C{H_3}} \right)_2}\mathop C\limits^ \bullet H < {\left( {C{H_3}} \right)_3}\mathop C\limits^ \bullet < {\left( {{C_6}{H_5}} \right)_2}\mathop C\limits^ \bullet H < {\left( {{C_6}{H_5}} \right)_3}\mathop C\limits^ \bullet $$
D
$${\left( {{C_6}{H_5}} \right)_3}\mathop C\limits^ \bullet < {\left( {{C_6}{H_5}} \right)_2}\mathop C\limits^ \bullet H < {\left( {C{H_3}} \right)_3}\mathop C\limits^ \bullet < {\left( {C{H_3}} \right)_2}\mathop C\limits^ \bullet H$$
Answer :
$${\left( {C{H_3}} \right)_2}\mathop C\limits^ \bullet H < {\left( {C{H_3}} \right)_3}\mathop C\limits^ \bullet < {\left( {{C_6}{H_5}} \right)_2}\mathop C\limits^ \bullet H < {\left( {{C_6}{H_5}} \right)_3}\mathop C\limits^ \bullet $$
260. Arrangement of $${\left( {C{H_3}} \right)_3}C - ,{\left( {C{H_3}} \right)_2}CH - ,C{H_3} - C{H_2} - $$ when attached to benzyl or an unsaturated group in increasing order of inductive effect is
A
$${\left( {C{H_3}} \right)_3}C - < {\left( {C{H_3}} \right)_2}CH - < C{H_3} - C{H_2}$$
B
$$C{H_3} - C{H_2} - < {\left( {C{H_3}} \right)_2}CH - < {\left( {C{H_3}} \right)_3}C - $$
C
$${\left( {C{H_3}} \right)_2}CH - < {\left( {C{H_3}} \right)_3}C - < C{H_3}, - C{H_2}$$
D
$${\left( {C{H_3}} \right)_3}C - < C{H_3} - C{H_2} - {\left( {C{H_3}} \right)_2}CH - $$
Answer :
$$C{H_3} - C{H_2} - < {\left( {C{H_3}} \right)_2}CH - < {\left( {C{H_3}} \right)_3}C - $$