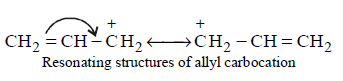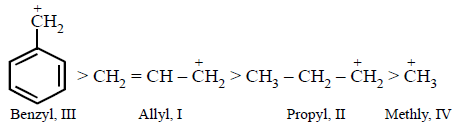241.
The order of stability of the following carbocations :
$$\eqalign{
& {\text{(I)}}C{H_2} = CH - \mathop C\limits^ + {H_2} \cr
& {\text{(II)}}C{H_3} - C{H_2} - \mathop C\limits^ + {H_2} \cr} $$
$${\text{(III)}}$$ 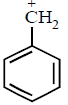
$${\text{(IV)}}\mathop C\limits^ + {H_3}\,{\text{is}}\,{\text{:}}$$
A
IV > III > II > I
B
II > III > I > IV
C
III > I > II > IV
D
III > I > IV > II
Answer :
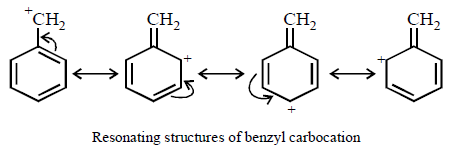
III > I > II > IV
242. Free radicals can undergo
A
rearrangement to a more stable free radical
B
decomposition to give another free radical
C
combination with other free radical
D
all are correct
Answer :
all are correct
243. Which of the following behaves both as a nucleophile and as an electrophile ?
A
$$C{H_3}C \equiv N$$
B
$$C{H_3}OH$$
C
$$C{H_2} = CHC{H_3}$$
D
$$C{H_3}N{H_2}$$
Answer :
$$C{H_3}C \equiv N$$
244.
In Carius method of estimation of halogens, $$250 mg$$ of an organic compound gave $$141 mg$$ of $$AgBr$$ . The percentage of bromine in the compound is :
$$\left( {{\text{at}}{\text{.}}\,{\text{mass}}\,Ag = 108;\,Br = 80} \right)$$
A
48
B
60
C
24
D
36
Answer :
24
245. Which of the following statements is not correct for a nucleophile?
A
Nucleophile is a Lewis acid
B
Ammonia is a nucleophile
C
Nucleophiles attack low electrons density sites
D
Nucleophiles are not electron seeking
Answer :
Nucleophile is a Lewis acid
246.
In the following carbocation, $$H/C{H_3}$$ that is most likely to migrate to the positively charged carbon is
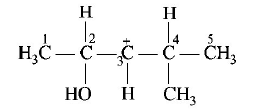
A
$$C{H_3}\,{\text{at}}\,C - 4$$
B
$$H\,{\text{at}}\,C - 4$$
C
$$C{H_3}\,{\text{at}}\,C - 2$$
D
$$H\,{\text{at}}\,C - 2$$
Answer :
$$H\,{\text{at}}\,C - 2$$
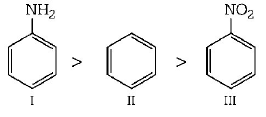
247. The correct order of reactivity towards the electrophilic substitution of the compounds aniline (I) benzene (II) and nitrobenzene (III) is
A
II < III > I
B
I > II > III
C
III > II > I
D
II > III > I
Answer :
I > II > III
248. The presence of carbon in an organic compound can be shown by
A
heating the compound with sodium
B
heating the compound with cupric oxide
C
heating the compound on Bunsen flame
D
heating the compound with magnesium
Answer :
heating the compound with cupric oxide
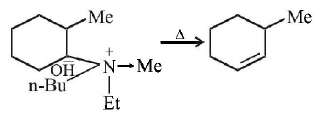
249.
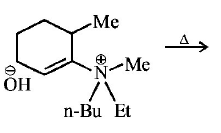
The alkene formed as a major product in the above
elimination reaction is
A
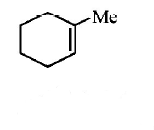

B
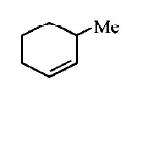

C
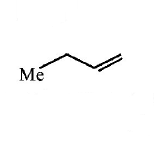

D
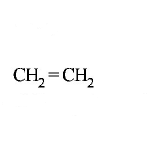
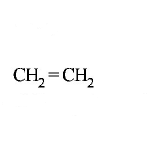
Answer :


250. For the estimation of nitrogen, $$1.4 g$$ of an organic compound was digested by Kjeldahl method and the evolved ammonia was absorbed in $$60\,{\text{mL}}\,{\text{of}}\,\frac{M}{{10}}$$ sulphuric acid. The unreacted acid required $$20\,{\text{mL}}\,{\text{of}}\,\frac{M}{{10}}$$ sodium hydroxide for complete neutralization. The percentage of nitrogen in the compound is:
A
$$6\% $$
B
$$10\% $$
C
$$3\% $$
D
$$5\% $$
Answer :
$$10\% $$