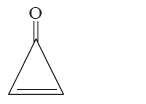
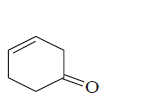
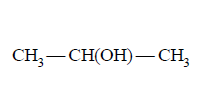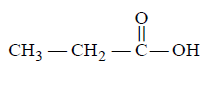201. Which of the following possesses a $$sp$$ - carbon in its structure?
A
$$C{H_2} = CCl - CH = C{H_2}$$
B
$$CC{l_2} = CC{l_2}$$
C
$$C{H_2} = C = C{H_2}$$
D
$$C{H_2} = CH - CH = C{H_2}$$
Answer :
$$C{H_2} = C = C{H_2}$$
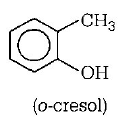
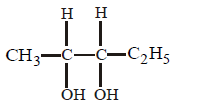
202. Which one of the following is most reactive towards electrophilic reagent?
A
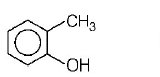

B


C


D


Answer :


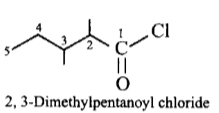
204.
The IUPAC name of  is
is
A
1-chloro-1-oxo-2, 3-dimethylpentane
B
2-ethyl-3-methylbutanoyl chloride
C
2, 3-dimethylpentanoyl chloride
D
3, 4-dimethylpentanoyl chloride
Answer :
2, 3-dimethylpentanoyl chloride
205. The IUPAC name of $${C_6}{H_5}COCl$$ is
A
Benzene chloro ketone
B
Benzoyl chloride
C
Chloro phenyl ketone
D
Benzene carbonyl chloride
Answer :
Benzoyl chloride
206. Which of the following is the correct IUPAC name?
A
3-Ethyl-4, 4-dimethylheptane
B
4, 4-Dimethyl-3-ethylheptane
C
5-Ethyl-4, 4-dimethylheptane
D
4, 4-Bis(methyl) -3-ethylheptane
Answer :
3-Ethyl-4, 4-dimethylheptane
207. Those substances can be separated by steam distillation are
A
steam volatile and insoluble in water
B
steam volatile and soluble in water
C
steam volatile and sparingly soluble in water
D
in liquid form in steam and solid form in water
Answer :
steam volatile and insoluble in water
208. Electronegativity of carbon atoms depends upon their state of hybridisation. In which of the following compounds, the carbon marked with asterisk is most electronegative?
A
$$C{H_3} - C{H_2} - \mathop C\limits^ * {H_2} - C{H_3}$$
B
$$C{H_3} - \mathop C\limits^ * H = CH - C{H_3}$$
C
$$C{H_3} - C{H_2} - C \equiv \mathop C\limits^ * H$$
D
$$C{H_3} - C{H_2} - CH = \mathop C\limits^ * {H_2}$$
Answer :
$$C{H_3} - C{H_2} - C \equiv \mathop C\limits^ * H$$
210. The compound 1, 2-butadiene has
A
only $$sp$$ hybridized carbon atoms
B
only $$s{p^2}$$ hybridized carbon atoms
C
both $$sp$$ and $$s{p^2}$$ hybridized carbon atoms
D
$$sp$$ , $$s{p^2}$$ and $$s{p^3}$$ hybridized carbon atoms
Answer :
$$sp$$ , $$s{p^2}$$ and $$s{p^3}$$ hybridized carbon atoms