71. Which of the following will not give butyl acetate when treated with 1- butanol
A


B
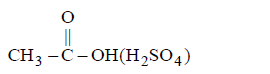
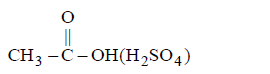
C
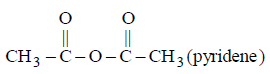
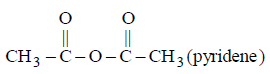
D


Answer :


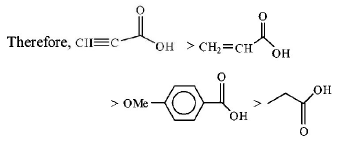
72.
The correct order of acid strength of the following carboxylic
acid is

A
I > II > III > IV
B
II > I > IV > III
C
I > III > II > IV
D
III > II > I > IV
Answer :
I > II > III > IV
73. The strongest acid amongst the following compounds is :
A
$$C{H_3}COOH$$
B
$$HCOOH$$
C
$$C{H_3}C{H_2}CH\left( {Cl} \right)C{O_2}H$$
D
$$ClC{H_2}C{H_2}COOH$$
Answer :
$$C{H_3}C{H_2}CH\left( {Cl} \right)C{O_2}H$$
74. The preparation of ethyl acetoacetate involves
A
Wittig reaction
B
Cannizaro’s reaction
C
Reformatsky reaction
D
Claisen condensation
Answer :
Claisen condensation
75. Which one of the following orders of acidic strength is correct ?
A
$$RCOOH > HOH > HC \equiv CH > ROH$$
B
$$RCOOH > HC \equiv CH > HOH > ROH$$
C
$$RCOOH > ROH > HOH > HC \equiv CH$$
D
$$RCOOH > HOH > ROH > HC \equiv CH$$
Answer :
$$RCOOH > HOH > ROH > HC \equiv CH$$
76.
The correct order of acidity for the following compounds is

A
I > II > III > IV
B
III > I > II > IV
C
III > IV > II > I
D
I > III > IV > II
Answer :
I > II > III > IV
77. Which one of the following esters gets hydrolysed most easily under alkaline conditions?
A


B
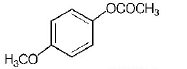

C


D


Answer :


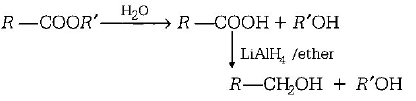
78. Reduction by $$LiAl{H_4}$$ of hydrolysed product of an ester gives
A
two acids
B
two aldehydes
C
one molecule of alcohol and another of carboxylic acid
D
two alcohols
Answer :
two alcohols
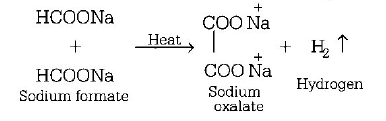
79. Sodium formate on heating yields.
A
Oxalic acid and $${H_2}$$
B
Sodium oxalate and $${H_2}$$
C
$$C{O_2}$$ and $$NaOH$$
D
Sodium oxalate
Answer :
Sodium oxalate and $${H_2}$$
80. Among acetic acid, phenol and $$n$$ - hexanol which one of the following compound will react with $$NaHC{O_3}$$ solution to give sodium salt and $$C{O_2}?$$
A
Acetic acid
B
$$n$$ - hexanol
C
Acetic acid and phenol
D
Phenol
Answer :
Acetic acid