21. The relative reactivities of acyl compounds towards nucleophilic substitution are in the order of
A
acyl chloride > acid anhydride > ester > amide
B
ester > acyl chloride > amide > acid anhydride
C
acid anhydride > amide > ester > acyl chloride
D
acyl chloride > ester > acid anhydride > amide
Answer :
acyl chloride > acid anhydride > ester > amide
22. On mixing ethyl acetate with aqueous sodium chloride, the composition of the resultant solution is
A
$$C{H_3}COCl + {C_2}{H_5}OH + NaOH$$
B
$$C{H_3}COONa + {C_2}{H_5}OH$$
C
$$C{H_3}COO{C_2}{H_5} + NaCl$$
D
$$C{H_3}Cl + {C_2}{H_5}COONa$$
Answer :
$$C{H_3}COO{C_2}{H_5} + NaCl$$
23.
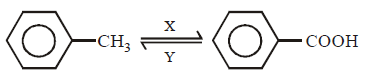
In the above sequence of reaction $$X$$ and $$Y$$ are respectively
A
\[{{H}_{2}}/Pt;B{{r}_{2}}\]
B
\[KMn{{O}_{4}};{{H}_{2}}/Pt\]
C
\[KMn{{O}_{3}}\left( aq \right);HI/P\]
D
\[N{{H}_{2}}-N{{H}_{2}}/KOH,HI/P\]
Answer :
\[KMn{{O}_{3}}\left( aq \right);HI/P\]
24.
Which lactone is formed by heating the following hydroxy acid ?

A


B


C


D


Answer :


25.
The correct order of acidity for the following compounds is

A
I > II > III > IV
B
III > I > II > IV
C
III > IV > II > I
D
I > III > IV > II
Answer :
I > II > III > IV
26. Carboxylic acid group does not give the usual addition and elimination reactions of aldehydes and ketones because
A
$$O–H$$ bond is more polar than  group
group
 group
group B
carboxylate ion gets ionised
C
carboxylate ion gets stabilised by resonance
D
it exists as $$-COOH$$ and there is no carbonyl group
Answer :
carboxylate ion gets stabilised by resonance
27. Compound \[Ph-O\overset{\begin{smallmatrix} O \\ \parallel \end{smallmatrix}}{\mathop{-C-}}\,Ph\] can be prepared by the reaction of ________.
A
phenol and benzoic acid in the presence of $$NaOH$$
B
phenol and benzoyl chloride in the presence of pyridine
C
phenol and benzoyl chloride in the presence of $$ZnC{l_2}$$
D
phenol and benzaldehyde in the presence of palladium
Answer :
phenol and benzoyl chloride in the presence of pyridine
28. Consider the following transformations \[C{{H}_{3}}COOH\xrightarrow{CaC{{O}_{3}}}A\xrightarrow{Heat}\] \[B\xrightarrow[NaOH]{{{I}_{2}}}C\] The molecular formula of $$C$$ is
A


B


C


D


Answer :


29. Which of the following orders of relative strengths of acids is correct ?
A
$$FC{H_2}COOH > ClC{H_2}COOH$$ $$ > BrC{H_2}COOH$$
B
$$ClC{H_2}COOH > BrC{H_2}COOH$$ $$ > FC{H_2}COOH$$
C
$$BrC{H_2}COOH > ClC{H_2}COOH$$ $$ > FC{H_2}COOH$$
D
$$ClC{H_2}C{O_2}H > FC{H_2}COOH$$ $$ > BrC{H_2}COOH$$
Answer :
$$FC{H_2}COOH > ClC{H_2}COOH$$ $$ > BrC{H_2}COOH$$
30.
The major product of the reaction is  \[\xrightarrow[{{0}^{\circ }}C]{NaN{{O}_{2}},\,\text{aqueous}\,HCl}\]
\[\xrightarrow[{{0}^{\circ }}C]{NaN{{O}_{2}},\,\text{aqueous}\,HCl}\]
A


B


C


D


Answer :







