81. Compound $${C_2}{H_6}O$$ has two isomers $$X$$ and $$Y.$$ On reaction with $$HI, X$$ gives alkyl iodide and water while $$Y$$ gives alkyl iodide and alcohol. Compounds $$X$$ and $$Y$$ are respectively
A
$${C_2}{H_5}O{C_2}{H_5}{\text{ and }}C{H_3}O{C_2}{H_5}$$
B
$$C{H_3}OC{H_3}{\text{ and }}{C_2}{H_5}OC{H_3}$$
C
$${C_2}{H_5}OH{\text{ and }}C{H_3}OC{H_3}$$
D
$$C{H_3}OH{\text{ and }}C{H_3}OC{H_3}$$
Answer :
$${C_2}{H_5}OH{\text{ and }}C{H_3}OC{H_3}$$
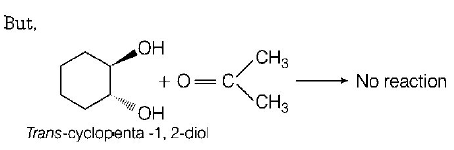
82. Which of the following reagents would distinguish $$cis$$ - cyclopenta -1, 2 - diol from the $$trans$$ - isomer?
A
Ozone
B
$$Mn{O_2}$$
C
Aluminium isopropoxide
D
Acetone
Answer :
Acetone
83.
Consider the following reaction,
\[\text{Phenol}\xrightarrow{Zn-\text{dust}}X\xrightarrow[\text{Anhy}\text{.}\,AlC{{l}_{3}}]{C{{H}_{3}}Cl}Y\xrightarrow{\begin{smallmatrix}
\text{Alk}\text{.} \\
KMn{{O}_{4}}
\end{smallmatrix}}Z\]
The product $$Z$$ is
A
toluene
B
benzaldehyde
C
benzoic acid
D
benzene
Answer :
benzoic acid
84. Thiol group is present in :
A
Cysteine
B
Methionine
C
Cytosine
D
Cystine
Answer :
Cysteine
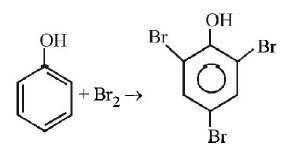
85. Phenol is heated with a solution of mixture of $$KBr$$ and$$KBr{O_3}.$$ The major product obtained in the above reaction is :
A
2 - Bromophenol
B
3 - Bromophenol
C
4 - Bromophenol
D
2, 4, 6 - Tribromophenol
Answer :
2, 4, 6 - Tribromophenol
86.
Sodium phenoxide when heated with $$C{O_2}$$ under pressure at
125°C yields a product which on acetylation produces $$C.$$

The major product C would be
A
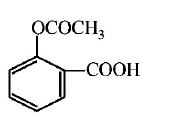

B
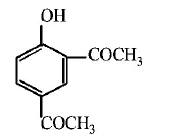

C
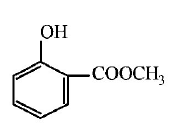

D
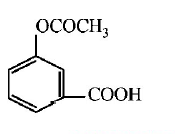

Answer :


87. The $$ - OH$$ group of an alcohol or the $$-COOH$$ group of a carboxylic acid can be replaced by $$-Cl$$ using
A
phosphorus pentachloride
B
hypochlorous acid
C
chlorine
D
hydrochloric acid
Answer :
phosphorus pentachloride
88. The correct sequence of decreasing acidity is
A
$${\left( {C{H_3}} \right)_3}COH > {\left( {C{H_3}} \right)_2}CHOH$$ $$ > {C_2}{H_5}OH > C{H_3}OH$$
B
$$C{H_3}OH > {C_2}{H_5}OH > {\left( {C{H_3}} \right)_2}CHOH$$ $$ > {\left( {C{H_3}} \right)_3}COH$$
C
$${C_2}{H_5}OH > C{H_3}OH > {\left( {C{H_3}} \right)_3}COH$$ $$ > {\left( {C{H_3}} \right)_2}CHOH$$
D
$${\left( {C{H_3}} \right)_2}CHOH > {\left( {C{H_3}} \right)_3}COH$$ $$ > {C_2}{H_5}OH > C{H_3}OH$$
Answer :
$$C{H_3}OH > {C_2}{H_5}OH > {\left( {C{H_3}} \right)_2}CHOH$$ $$ > {\left( {C{H_3}} \right)_3}COH$$
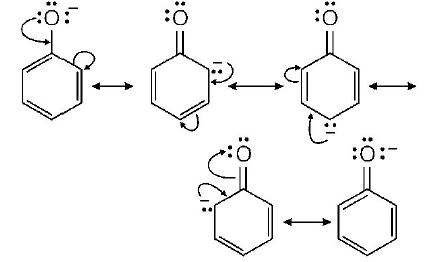
89. The ionisation constant of phenol is higher than that of ethanol because
A
phenoxide ion is bulkier than ethoxide
B
phenoxide ion is stronger base than ethoxide
C
phenoxide ion is stabilised through delocalisation
D
phenoxide ion is less stable than ethoxide
Answer :
phenoxide ion is stabilised through delocalisation
90. For the identification of $$\beta $$ - naphthol using dye test, it is necessary to use
A
Dichloromethane solution of $$\beta $$ - naphthol
B
Acidic solution of $$\beta $$ - naphthol
C
Neutral solution of $$\beta $$ - naphthol
D
Alkaline solution of $$\beta $$ - naphthol
Answer :
Alkaline solution of $$\beta $$ - naphthol