41. A compound of the formula $${C_4}{H_{10}}O$$ reacts with sodium and undergoes oxidation to give a carbonyl compound which does not reduce Tollen’s reagent, the original compound is
A
Diethyl ether
B
$$n$$ - Butyl alcohol
C
Isobutyl alcohol
D
$$sec$$ - Butyl alcohol
Answer :
$$sec$$ - Butyl alcohol
43. Identity $$Z$$ in the sequence of reactions, \[C{{H}_{3}}C{{H}_{2}}CH=C{{H}_{2}}\xrightarrow{\frac{HBr}{{{H}_{2}}{{O}_{2}}}}\] \[Y\xrightarrow{{{C}_{2}}{{H}_{5}}ONa}Z\]
A
$$C{H_3} - {\left( {C{H_2}} \right)_3} - O - C{H_2}C{H_3}$$
B
$${\left( {C{H_3}} \right)_2}C{H_2} - O - C{H_2}C{H_3}$$
C
$$C{H_3}{\left( {C{H_2}} \right)_4} - O - C{H_3}$$
D
$$C{H_3}C{H_2} - CH\left( {C{H_3}} \right) - O - C{H_2}C{H_3}$$
Answer :
$$C{H_3} - {\left( {C{H_2}} \right)_3} - O - C{H_2}C{H_3}$$
45. Which of the following alcohols is dehydrated most easily with conc. $${H_2}S{O_4}?$$
A
$$p - {O_2}N{C_6}{H_4}CH\left( {OH} \right)C{H_3}$$
B
$$p - Cl{C_6}{H_4}CH\left( {OH} \right)C{H_3}$$
C
$$p - C{H_3}O{C_6}{H_4}CH\left( {OH} \right)C{H_3}$$
D
$${C_6}{H_5}CH\left( {OH} \right)C{H_3}$$
Answer :
$$p - C{H_3}O{C_6}{H_4}CH\left( {OH} \right)C{H_3}$$
46. Out of 2-chloroethanol and ethanol which is more acidic and why?
A
2-Chloroethanol due to $$+ I$$ effect of $$Cl$$
B
Ethanol due to $$+ I$$ effect of $$C{H_3}$$
C
2-Chloroethanol due to $$- I$$ effect of $$Cl$$
D
Ethanol due to $$- I$$ effect of $$C{H_3}$$
Answer :
2-Chloroethanol due to $$- I$$ effect of $$Cl$$
47.
Match the column I with column II and mark the appropriate choice.
Column I
Column II
a.
Williamson's synthesis
1.
$${C_6}{H_5}OH + C{H_3}COCl$$ in presence of pyridine
b.
$$ROR'$$
2.
$${C_2}{H_5}ONa + {C_2}{H_5}Br$$
c.
$$p$$ - Nitrophenol
3.
Unsymmetrical ether
d.
Acetylation
4.
Intermolecular hydrogen bonding
A
a - 1, b - 3, c - 2, d - 4
B
a - 3, b - 1, c - 2, d - 4
C
a - 2, b - 3, c - 4, d - 1
D
a - 4, b - 1, c - 2, d - 3
Answer :
a - 2, b - 3, c - 4, d - 1
48. The best reagent to convert pent $$- 3 - en - 2 - ol$$ into pent $$- 3 - in - 2 - one$$ is
A
Pyridinium chloro - chromate
B
Chromic anhydride in glacial acetic acid
C
A acidic dichromate
D
Acidic permanganate
Answer :
Pyridinium chloro - chromate
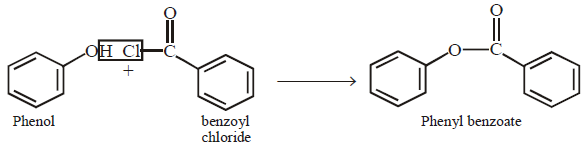
49. The reaction of phenol with benzoyl chloride to give phenyl benzoate is known as :
A
Claisen reaction
B
Schotten - Baumann reaction
C
Reimer - Tiemann reaction
D
Gatterman-Koch reaction
Answer :
Schotten - Baumann reaction
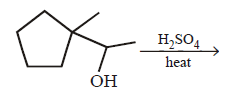
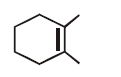
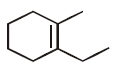
50.
Identify the major product, 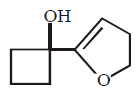 \[\xrightarrow{{{H}^{+}}}\] Product
\[\xrightarrow{{{H}^{+}}}\] Product
A
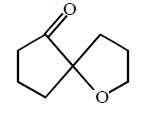

B


C
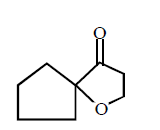

D

Answer :