31. Which of the following compounds will react with sodium hydroxide solution in water?
A
$${C_6}{H_5}OH$$
B
$${C_6}{H_5}C{H_2}OH$$
C
$${\left( {C{H_3}} \right)_3}COH$$
D
$${C_2}{H_5}OH$$
Answer :
$${C_6}{H_5}OH$$
32. Which of the following are the products shown by the reaction of methoxyethane with $$HI?$$
A
$${C_2}{H_5}I + C{H_3}OH$$
B
$$C{H_3}I + {H_2}O$$
C
$${C_2}{H_5}OH + {H_2}O$$
D
$${C_2}{H_5}OH + C{H_3}I$$
Answer :
$${C_2}{H_5}OH + C{H_3}I$$
33. The compound which reacts fastest with Lucas reagent at room temperature is
A
$${\text{butan - 1 - ol}}$$
B
$${\text{butan - 2 - ol}}$$
C
$${\text{2 - methylpropan - 1 - ol}}$$
D
$${\text{2 - methylpropan - 2 - ol}}$$
Answer :
$${\text{2 - methylpropan - 2 - ol}}$$
34. Acid catalysed dehydration of $$t$$ - butanol is faster than that of $$n$$ - butanol because
A
tertiary carbocation is more stable than primary carbocation
B
primary carbocation is more stable than tertiary carbocation
C
$$t$$ - butanol has a higher boiling point
D
rearrangement takes place during dehydration of $$t$$ - butanol.
Answer :
tertiary carbocation is more stable than primary carbocation
35. $$ortho$$ - Nitrophenol is less soluble in water than $$p$$ - and $$m$$ - nitrophenols because
A
$$o$$ - nitrophenol shows intramolecular $$H$$ - bonding
B
$$o$$ - nitrophenol shows intermolecular $$H$$ - bonding
C
melting point of $$o$$ - nitrophenol is lower than those of $$m$$ - and $$p$$ -isomers
D
$$o$$ - nitrophenol is more volatile in steam than those of $$m$$ - and $$p$$ -isomers
Answer :
$$o$$ - nitrophenol shows intramolecular $$H$$ - bonding
36.
A compound $$X\left( {{C_8}{H_{10}}O} \right)$$ upon treatment with alkaline solution of iodine gives a yellow precipitate. The filtrate on acidification gives a white solid $$Y\left( {{C_7}{H_6}{O_2}} \right).$$ Write the structures of $$X$$ and $$Y.$$

A
(a)
B
(b)
C
(c)
D
(d)
Answer :
(b)
37. The best reagent to convert pent-3-en-2-ol into pent-3-en-2-one is
A
acidic permanganate
B
acidic dichromate
C
chromic anhydride in glacial acetic acid
D
pyridinium chlorochromate
Answer :
pyridinium chlorochromate
38.
Identify the nature of product in the following reaction
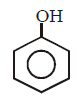 \[+{{K}_{2}}{{S}_{2}}{{O}_{8}}\xrightarrow[{{H}_{2}}O]{H{{O}^{-}}}\text{Product}\]
\[+{{K}_{2}}{{S}_{2}}{{O}_{8}}\xrightarrow[{{H}_{2}}O]{H{{O}^{-}}}\text{Product}\]
A


B
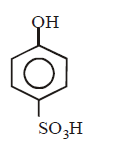

C


D


Answer :


39. In Williamson synthesis of mixed ether having a primary and a tertiary alkyl group if tertiary halide is used, then :
A
Rate of reaction will be slow due to slow cleavage of carbon-halogen bond.
B
Alkene will be the main product.
C
Simple ether will form instead of mixed ether.
D
Expected mixed ether will be formed.
Answer :
Alkene will be the main product.
40. Which of the following compounds does not react with $$NaOH?$$
A
$$C{H_3}COOH$$
B
$$C{H_3}CON{H_2}$$
C
$${C_6}{H_5}OH$$
D
$$C{H_3}C{H_2}OH$$
Answer :
$$C{H_3}C{H_2}OH$$

.PNG)
