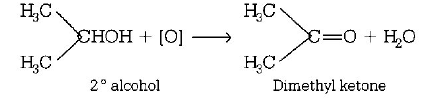
21. Which one of the following on oxidation gives a ketone?
A
Primary alcohol
B
Secondary alcohol
C
Tertiary alcohol
D
All of these
Answer :
Secondary alcohol
22. How many isomers of $${C_5}{H_{11}}OH$$ will be primary alcohols?
A
5
B
4
C
2
D
3
Answer :
4
23.
Arrange the following alcohols in order of increasing reactivity towards sodium metal.
$$\eqalign{
& \left( {\text{i}} \right){\left( {C{H_3}} \right)_3}C - OH \cr
& \left( {{\text{ii}}} \right){\left( {C{H_3}} \right)_2}CH - OH \cr
& \left( {{\text{iii}}} \right)C{H_3}C{H_2}OH \cr} $$
A
(iii) < (ii) < (i)
B
(ii) < (i) < (iii)
C
(i) < (ii) > (iii)
D
(iii) < (i) < (ii)
Answer :
(i) < (ii) > (iii)
24. The enzyme which can catalyse the conversion of glucose to ethanol is
A
invertase
B
zymase
C
maltase
D
diastase
Answer :
zymase
25.
Arrange the following in increasing order of their acidity ?
(a) $$o-$$ cresol, (b) salicylic acid, (c) phenol
A
c < a < b
B
b < c < a
C
a < b < a
D
a < c < b
Answer :
a < c < b
26.
Consider the following reaction sequence,
\[C{{H}_{3}}CH\left( OH \right)C{{H}_{3}}\xrightarrow[\text{Heat}]{\text{Conc}.\,{{H}_{2}}S{{O}_{4}}}\] \[X\xrightarrow[{{H}_{2}}O]{\text{dil}\text{.}\,{{H}_{2}}S{{O}_{4}}}Y\]
$$X$$ and $$Y$$ in the reaction respectively are
A
$$C{H_3}CH = C{H_2},C{H_3}CH\left( {OH} \right)C{H_3}$$
B
$$C{H_3}CH = CHC{H_3},C{H_3}C{H_2}C{H_2}OH$$
C
$$C{H_3}C{H_2}CH = C{H_2},C{H_3}C{H_2}C{H_2}OH$$
D
$$C{H_3}C{H_2}C{H_2}C{H_3},$$ $$C{H_3}CH\left( {OH} \right)C{H_2}C{H_3}$$
Answer :
$$C{H_3}CH = C{H_2},C{H_3}CH\left( {OH} \right)C{H_3}$$
27.
Diethyl ether when refluxed with excess of $$HI$$ gives two molecules of $$\underline {\,\,\left( {\text{i}} \right)\,\,} .$$ Ethers can be most commonly prepared by reaction of $$\underline {\,\,\left( {{\text{ii}}} \right)\,\,} $$ and $$\underline {\,\,\left( {{\text{iii}}} \right)\,\,} .$$ The method is called $$\underline {\,\,\left( {{\text{iv}}} \right)\,\,} .$$
(i), (ii), (iii) and (iv) respectively are
A
ethyl iodide, sodium alkoxide, alkyl halide, Williamson's synthesis
B
ethanol, alcohol, alkyl halide, substitution
C
methyl iodide, Grignards reagent, alkyl halide, Williamson's synthesis
D
ethyl iodide, phenol, ethyl iodide, esterification
Answer :
ethyl iodide, sodium alkoxide, alkyl halide, Williamson's synthesis
28. During dehydration of alcohols to alkenes by heating with conc. \[{{H}_{2}}S{{O}_{4}}\] the initiation step is
A
formation of carbocation
B
elimination of water
C
formation of an ester
D
protonation of alcohol molecule
Answer :
protonation of alcohol molecule
29.
Diols ( I - IV ) which react with \[Cr{{O}_{3}}\] in aqueous \[{{H}_{2}}S{{O}_{4}}\] and yield products that readily under go dercarboxylation on heating, are :

A
I and II
B
II and III
C
II and IV
D
I and IV
Answer :
II and IV
30. Benzoquinone is prepared by reaction of phenol with
A
$$N{a_2}C{r_2}{O_7},{H_2}S{O_4}$$
B
$$KMn{O_4},{H_2}S{O_4}$$
C
$$N{a_2}Cr{O_4},HCl$$
D
$${K_2}Mn{O_4},{H_2}S{O_4}$$
Answer :
$$N{a_2}C{r_2}{O_7},{H_2}S{O_4}$$