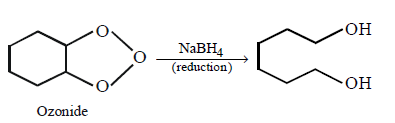
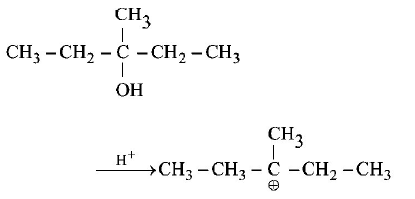
252.
Succinic acid
\[\xrightarrow{\Delta }\left( A \right)\xrightarrow[\Delta ]{N{{H}_{3}}}\left( B \right)\xrightarrow[KOH]{B{{r}_{2}}}\left( C \right);\]
Product $$(C)$$ will be :
A


B
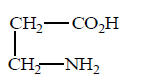
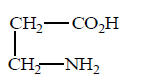
C


D


Answer :


253. Among the following compounds which can be dehydrated very easily is
A
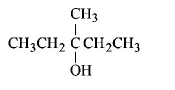
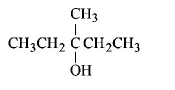
B
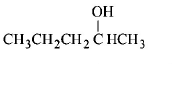
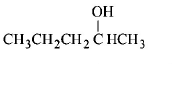
C


D
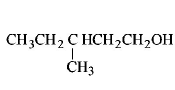
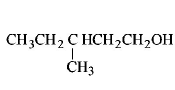
Answer :
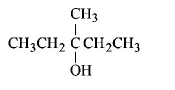
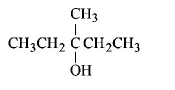
254. Rate of dehydration of alcohols follows the order :
A
$${2^ \circ } > {1^ \circ } > C{H_3}OH > {3^ \circ }$$
B
$${3^ \circ } > {2^ \circ } > {1^ \circ } > C{H_3}OH$$
C
$${2^ \circ } > {3^ \circ } > {1^ \circ } > C{H_3}OH$$
D
$$C{H_3}OH > {1^ \circ } > {2^ \circ } > {3^ \circ }$$
Answer :
$${3^ \circ } > {2^ \circ } > {1^ \circ } > C{H_3}OH$$
255. An organic compound with molecular formula $${C_4}{H_{10}}O$$ does not react with sodium. With excess of $$HI$$ it gives only one type of alkyl halide. The compound is
A
$${C_2}{H_5}O{C_2}{H_5}$$
B
\[\underset{\begin{smallmatrix}
|\,\,\,\,\,\,\, \\
\,OC{{H}_{3}}
\end{smallmatrix}}{\mathop{C{{H}_{3}}CHC{{H}_{3}}}}\,\]
C
$$C{H_3}C{H_2}C{H_2}OC{H_3}$$
D
$$C{H_3}C{H_2}C{H_2}C{H_2}OH$$
Answer :
$${C_2}{H_5}O{C_2}{H_5}$$
256. An alkene $$C{H_3}CH = C{H_2}$$ is treated with $${B_2}{H_6}$$ in presence of $${H_2}{O_2}.$$ The final product formed is
A
$$C{H_3}C{H_2}CHO$$
B
$$C{H_3}CH\left( {OH} \right)C{H_3}$$
C
$$C{H_3}C{H_2}C{H_2}OH$$
D
$${\left( {C{H_3}C{H_2}C{H_2}} \right)_3}B$$
Answer :
$$C{H_3}C{H_2}C{H_2}OH$$
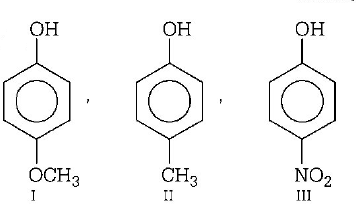
257. Increasing order of acidic strength among $$p$$ - methoxy phenol (I), $$p$$ - methyl phenol (II) and $$p$$ - nitrophenol (III) is
A
III, I, II
B
II, I, III
C
III, II, I
D
I, II, III
Answer :
I, II, III
258. Boiling point of ethyl alcohol is greater than ether due to
A
van der Waals forces
B
London forces
C
polarity
D
hydrogen bonding
Answer :
hydrogen bonding
259.
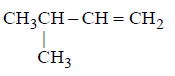 \[\xrightarrow[\left( \text{ii} \right)\,{{H}_{2}}{{O}_{2}}/O{{H}^{-}}]{\left( \text{i} \right)\,{{B}_{2}}{{H}_{6}}}X\xrightarrow[{{140}^{\circ }}C]{{{H}_{2}}S{{O}_{4}}}Y.\] What is $$Y ?$$
\[\xrightarrow[\left( \text{ii} \right)\,{{H}_{2}}{{O}_{2}}/O{{H}^{-}}]{\left( \text{i} \right)\,{{B}_{2}}{{H}_{6}}}X\xrightarrow[{{140}^{\circ }}C]{{{H}_{2}}S{{O}_{4}}}Y.\] What is $$Y ?$$
A


B
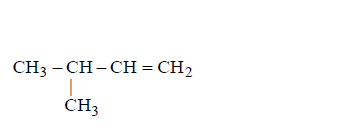
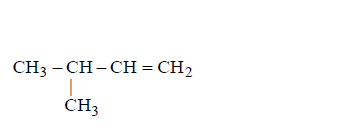
C


D


Answer :


260. For the reaction, $${C_2}{H_5}OH + HX \to {C_2}{H_5}X + {H_2}O;$$ the order of reactivity is
A
$$HCl > HBr > HI$$
B
$$HI> HBr > HCl$$
C
$$HBr > HCl > HI$$
D
$$HI > HCl > HBr$$
Answer :
$$HI> HBr > HCl$$
 Product is
Product is