191. An industrial method of preparation of methanol is :
A
catalytic reduction of carbon monoxide in presence of $$ZnO - C{r_2}{O_3}$$
B
by reacting methane with steam at 900°C with a nickel catalyst
C
by reducing formaldehyde with lithium aluminium hydride .
D
by reacting formaldehyde with aqueous sodium hydroxide solution
Answer :
catalytic reduction of carbon monoxide in presence of $$ZnO - C{r_2}{O_3}$$
192. What is the structure of the major product when phenol is treated with bromine water ?
A


B

C


D


Answer :


193. Vapours of an alcohol $$X$$ when passed over hot reduced copper, produce an alkene, the alcohol is
A
primary alcohol
B
secondary alcohol
C
tertiary alcohol
D
dihydric alcohol
Answer :
tertiary alcohol
194. How many isomers of $${C_5}{H_{11}}OH$$ will be primary alcohols ?
A
5
B
4
C
2
D
3
Answer :
4
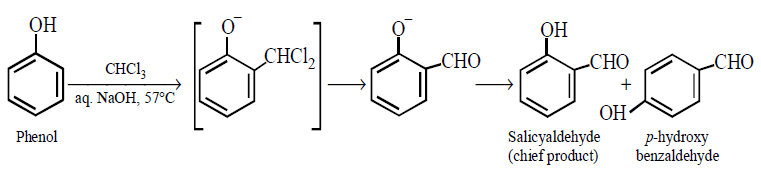
195. Phenol on heating with $$CHC{l_3}$$ and $$NaOH$$ gives salicylaldehyde. The reaction is called :
A
Reimer - Tiemann reaction
B
Claisen reaction
C
Cannizzaro’s reaction
D
Hell - Volhard - Zelinsky reaction
Answer :
Reimer - Tiemann reaction
196. Anisole on reaction with chloromethane in presence of anhydrous $$AlC{l_3}$$ gives
A
$$o$$ - methyl anisole and $$p$$ - methoxy anisole
B
$$p$$ - methyl anisole and $$p$$ - methoxy anisole
C
$$o$$ - methyl anisole and $$p$$ - methyl anisole
D
$$o$$ - methoxy acetophenone and $$p$$ - methoxy acetophenone.
Answer :
$$o$$ - methyl anisole and $$p$$ - methyl anisole
197.
Mark the correct increasing order of reactivity of the following compounds with $$HBr/HCl.$$

A
(i) < (ii) < (iii)
B
(ii) < (i) < (iii)
C
(ii) < (iii) < (i)
D
(iii) < (ii) < (i)
Answer :
(ii) < (iii) < (i)
198.
What is the major product of the following reaction ?

\[\xrightarrow[\text{Pyridine cold}]{Cr{{O}_{3}}}\text{Product}\]
A


B


C


D


Answer :


199. Which of the following compound can react with hydroxylamine ?
A

B
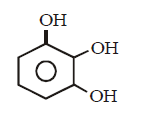

C
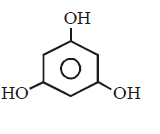

D
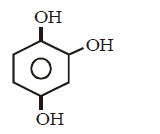

Answer :


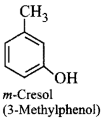
200. IUPAC name of $$m$$ - cresol is
A
3-methylphenol
B
3-chlorophenol
C
3-methoxyphenol
D
benzene-1, 3-diol
Answer :
3-methylphenol