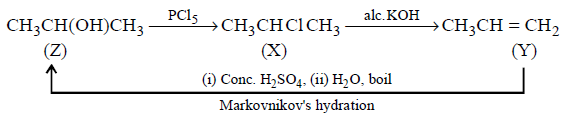161.
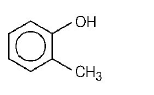
Mark the correct order of decreasing acid strength of the following compounds.
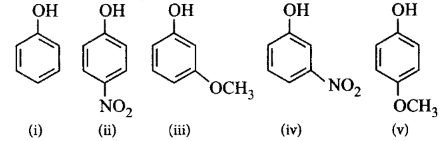
A
(v) > (iv) > (ii) > (i) > (iii)
B
(ii) > (iv) > (i) > (iii) > (v)
C
(iv) > (v) > (iii) > (ii) > (i)
D
(v) > (iv) > (iii) > (ii) > (i)
Answer :
(ii) > (iv) > (i) > (iii) > (v)
162. Which of the following reagents cannot be used to oxidise primary alcohols to aldehydes?
A
$$Cr{O_3}$$ in anhydrous medium
B
$$KMn{O_4}$$ in acidic medium
C
Pyridinium chlorochromate
D
Heat in the presence of $$Cu$$ at $$573\,K$$
Answer :
$$KMn{O_4}$$ in acidic medium
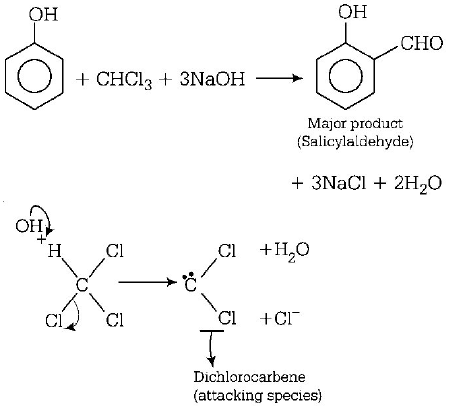
163. When phenol is treated with $$CHC{l_3}$$ and $$NaOH$$ the product formed is
A
benzaldehyde
B
salicylaldehyde
C
salicylic acid
D
benzoic acid
Answer :
salicylaldehyde
165. An organic compound $$A$$ reacts with methyl magnesium iodide to form an addition product which on hydrolysis forms the compound $$B.$$ Compound $$B$$ gives blue colour salt in Victor Meyer’s test. The compounds $$A$$ and $$B$$ are respectively
A
acetaldehyde, tertiary butyl alcohol
B
acetaldehyde, ethyl alcohol
C
acetaldehyde, isopropyl alcohol
D
acetone, isopropyl alcohol
Answer :
acetaldehyde, isopropyl alcohol
166.
\[{{\left( C{{H}_{3}} \right)}_{3}}C-C{{H}_{2}}OH\xrightarrow[{{170}^{\circ }}C]{\text{Conc}.\,{{H}_{2}}S{{O}_{4}}}X\]
In the reaction, $$X$$ is
A
$${\left( {C{H_3}} \right)_2}C = CHC{H_3}$$
B
$$C{H_3}C \equiv CH$$
C
$${\left( {C{H_3}} \right)_2}CHC{H_2}C{H_3}$$
D
\[C{{H}_{3}}-C{{H}_{2}}\underset{\begin{smallmatrix}
|\,\, \\
\,\,C{{H}_{3}}
\end{smallmatrix}}{\mathop{-C=}}\,C{{H}_{2}}\]
Answer :
$${\left( {C{H_3}} \right)_2}C = CHC{H_3}$$
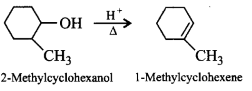
167. The major product of acid catalysed dehydration of 2-methylcyclohexanol and butan-1-ol are respectively
A
1-methylcyclohexene and but-1-ene
B
2-methylcyclohexene and but-2-ene
C
2-methylcyclohexene and butane
D
1-methylcyclohexene and but-2-ene
Answer :
1-methylcyclohexene and but-1-ene
168. The decreasing order of boiling points of the following alcohols is
A
3-methylbutan-2-ol > 2-methylbutan-2-ol > pentan-1-ol
B
pentan-1-ol > 3-methylbutan-2-ol > 2-methylbutan-2-ol
C
2-methylbutan-2-ol > 3-methylbutan-2-ol > pentan-1-ol
D
2-methylbutan-2-ol > pentan-1-ol > 3-methylbutan-2-ol
Answer :
pentan-1-ol > 3-methylbutan-2-ol > 2-methylbutan-2-ol
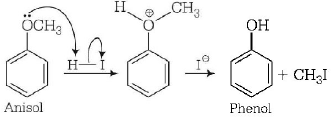
169. The heating of phenyl-methyl ethers with $$HI$$ produces.
A
ethyl chlorides
B
iodobenzene
C
phenol
D
benzene
Answer :
phenol
170.
What is $$Z$$ in the following sequence of reactions ?
\[Z\xrightarrow{PC{{l}_{5}}}X\xrightarrow{Alc.\,KOH}Y\] \[\xrightarrow[\left( \text{ii} \right)\,{{H}_{2}}O;\,\text{boil}]{\left( \text{i} \right)\,Conc.\,{{H}_{2}}S{{O}_{4}}}Z\]
A
$$C{H_3}C{H_2}C{H_2}OH$$
B
$$C{H_3}CH\left( {OH} \right)C{H_3}$$
C
$${\left( {C{H_3}C{H_2}} \right)_2}CHOH$$
D
$$C{H_3}CH = C{H_2}$$
Answer :
$$C{H_3}CH\left( {OH} \right)C{H_3}$$