471.
Match the interhalogen compounds of Column I with the geometry in Column II and assign the correct code.
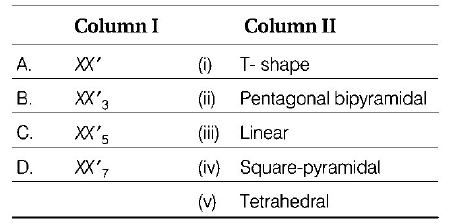
Code
$$\eqalign{
& \,\,\,\,\,\,\,\,\,\,\,\,\,{\text{A}}\,\,\,\,\,\,\,\,\,{\text{B}}\,\,\,\,\,\,\,\,\,\,{\text{C}}\,\,\,\,\,\,{\text{D}} \cr
& \left( {\text{a}} \right)\,\,\left( {{\text{iii}}} \right)\,\,\,\,\left( {{\text{iv}}} \right)\,\,\,\,\left( {\text{i}} \right)\,\,\,\,\left( {{\text{ii}}} \right) \cr
& \left( {\text{b}} \right)\,\,\left( {{\text{iii}}} \right)\,\,\,\,\,\left( {\text{i}} \right)\,\,\,\,\left( {{\text{iv}}} \right)\,\,\,\left( {{\text{ii}}} \right) \cr
& \left( {\text{c}} \right)\,\,\,\left( {\text{v}} \right)\,\,\,\,\,\left( {{\text{iv}}} \right)\,\,\,\left( {{\text{iii}}} \right)\,\,\left( {{\text{ii}}} \right) \cr
& \left( {\text{d}} \right)\,\,\left( {{\text{iv}}} \right)\,\,\,\,\left( {{\text{iii}}} \right)\,\,\,\left( {{\text{ii}}} \right)\,\,\,\,\left( {\text{i}} \right) \cr} $$
A
(a)
B
(b)
C
(c)
D
(d)
Answer :
(b)
472.
Which compound is prepared by the following reaction?
\[\underset{\left( 2:1\,\text{volume ratio} \right)}{\mathop{Xe+{{F}_{2}}}}\,\xrightarrow[673\,K]{Ni}\]
A
$$Xe{F_4}$$
B
$$Xe{F_2}$$
C
$$Xe{F_6}$$
D
$${\text{None of these}}$$
Answer :
$$Xe{F_2}$$
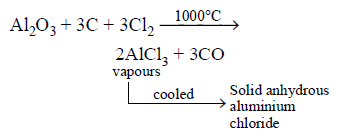
473. $$A{l_2}{O_3}$$ can be converted to anhydrous $$AlC{l_3}$$ by heating
A
$$A{l_2}{O_3}$$ with $$NaCl$$ in solid state
B
a mixture of $$A{l_2}{O_3}$$ and carbon in dry $$C{l_2}$$ gas
C
$$A{l_2}{O_3}$$ with $$C{l_2}$$ gas
D
$$A{l_2}{O_3}$$ with $$HCl$$ gas
Answer :
a mixture of $$A{l_2}{O_3}$$ and carbon in dry $$C{l_2}$$ gas
474. The melting $$pt.$$ of group 13 follows the order
A
$$B > Al > Ga > In > Tl$$
B
$$B > Al < Ga > In > Tl$$
C
$$B > Al > Tl > In > Ga$$
D
$$B > Al < Ga < In < Tl$$
Answer :
$$B > Al > Tl > In > Ga$$
475. In which of the following the inert pair effect is most prominent?
A
$$C$$
B
$$Ge$$
C
$$Si$$
D
$$Pb$$
Answer :
$$Pb$$
476. Concentrated $${H_2}S{O_4}$$ is not used to prepare $$HBr$$ from $$KBr$$ because it
A
oxidizes $$HBr.$$
B
reduces $$HBr.$$
C
causes disproportionation of $$HBr.$$
D
reacts too slowly with $$KBr.$$
Answer :
oxidizes $$HBr.$$
477. The bleaching action of chlorine is due to
A
reduction
B
hydrogenation
C
chloronation
D
oxidation
Answer :
oxidation
478.
$$B{\left( {OH} \right)_3} + NaOH \to NaB{O_2} + Na\left[ {B{{\left( {OH} \right)}_4}} \right] + {H_2}O$$
How can this reaction is made to proceed in forward direction ?
A
addition of $$cis\,1,2{\text{ - }}diol$$
B
addition of borax
C
addition of trans $$1,2{\text{ - }}diol$$
D
addition of $$N{a_2}HP{O_4}$$
Answer :
addition of $$cis\,1,2{\text{ - }}diol$$
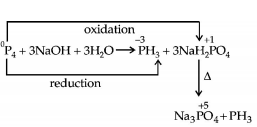
479. The reaction of white phosphorus with aqueous $$NaOH$$ gives phosphine along with another phosphorus containing compound. The reaction type; the oxidation states of phosphorus in phosphine and the other product are respectively
A
redox reaction; $$\, - 3\,\,{\text{and}}\, - 5$$
B
redox reaction; $$ + 3\,\,{\text{and}}\, + 5$$
C
disproportionation reaction; $$ - 3\,\,{\text{and}}\, + 5$$
D
disproportionation reaction; $$ - 3\,\,{\text{and}}\, + 3\,$$
Answer :
disproportionation reaction; $$ - 3\,\,{\text{and}}\, + 5$$
480. The products obtained when chlorine gas reacts with cold and dilute acqueous $$NaOH$$ are:
A
$$Cl{O^ - }\,{\text{and}}\,ClO_3^ - $$
B
$$ClO_2^ - \,{\text{and}}\,ClO_3^ - $$
C
$$C{l^ - }\,{\text{and}}\,Cl{O^ - }$$
D
$$C{l^ - }\,{\text{and}}\,ClO_2^ - $$
Answer :
$$C{l^ - }\,{\text{and}}\,Cl{O^ - }$$