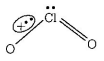
461. Ammonia can be dried by
A
$${\text{conc}}{\text{.}}\,{H_2}S{O_4}$$
B
$${P_4}{O_{10}}$$
C
$$CaO$$
D
$${\text{anhydrous}}\,CaC{l_2}$$
Answer :
$$CaO$$
462. Which one of the following oxides is expected to exhibit paramagnetic behaviour?
A
$$C{O_2}$$
B
$$S{O_2}$$
C
$$Cl{O_2}$$
D
$$Si{O_2}$$
Answer :
$$Cl{O_2}$$
463. A deep brown gas is formed by mixing two colourless gases which are
A
$$N{O_2}\,\,{\text{and}}\,\,{O_2}$$
B
$${N_2}O\,\,{\text{and}}\,\,NO$$
C
$$NO\,\,{\text{and}}\,\,{O_2}$$
D
$$N{H_3}\,\,{\text{and}}\,\,HCl$$
Answer :
$$NO\,\,{\text{and}}\,\,{O_2}$$
464. Dry ice is
A
$${\text{solid}}\,N{H_3}$$
B
$${\text{solid}}\,S{O_2}$$
C
$${\text{solid}}\,C{O_2}$$
D
$${\text{solid}}\,{N_2}$$
Answer :
$${\text{solid}}\,C{O_2}$$
465. Brown colour in $$HN{O_3}$$ can be removed by
A
adding $$Mg$$ powder.
B
boiling the acid.
C
passing $$N{H_3}$$ through acid.
D
passing air through warm acid.
Answer :
passing air through warm acid.
466. Lead pipes are not suitable for drinking water because
A
lead forms basic lead carbonate
B
lead reacts with water containing air to form $$Pb{\left( {OH} \right)_2}$$
C
a layer of lead dioxide is deposited over pipes
D
lead reacts with air to form litharge
Answer :
lead reacts with water containing air to form $$Pb{\left( {OH} \right)_2}$$
467. Excess of $$KI$$ reacts with $$CuS{O_4}$$ solution and then $$N{a_2}{S_2}{O_3}$$ solution is added to it. Which of the statements is incorrect for this reaction ?
A
$$N{a_2}{S_2}{O_3}$$ is oxidised
B
$$Cu{I_2}$$ is formed
C
$$C{u_2}{I_2}$$ is formed
D
Evolved $${I_2}$$ is reduced
Answer :
$$Cu{I_2}$$ is formed
468. Choose the correct statements from the following.
A
Rhombic sulphur is blue in colour.
B
Rhombic sulphur is soluble in water but insoluble in organic solvents.
C
Rhombic and monoclinic sulphur have $${S_6}$$ molecules.
D
In cycle - $${S_6}$$ molecule, the ring adopts chair form.
Answer :
In cycle - $${S_6}$$ molecule, the ring adopts chair form.
469. Which of the following is not a use of noble gases?
A
Argon is widely used for filling incandescent electric bulbs.
B
Neon is used in safety devices for protecting electrical instruments.
C
Radon is used in radiotherapy of cancer.
D
Helium is filled in tubes of cycles and scooters tyres.
Answer :
Helium is filled in tubes of cycles and scooters tyres.
470. Nitrogen is relatively inactive element because
A
its atoms has a stable electronic configuration
B
it has low atomic radius
C
its electronegativity is fairly high
D
dissociation energy of its molecule is fairly high
Answer :
dissociation energy of its molecule is fairly high