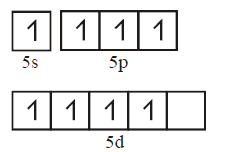
441. $$Xe{O_4}$$ molecule is tetrahedral having :
A
Two $$p\pi - d\pi $$ bonds
B
One $$p\pi - d\pi $$ bonds
C
Four $$p\pi - d\pi $$ bonds
D
Three $$p\pi - d\pi $$ bonds
Answer :
Four $$p\pi - d\pi $$ bonds
442. It is possible to obtain oxygen from air by fractional distillation because
A
oxygen is in a different group of the periodic table from nitrogen
B
oxygen is more reactive than nitrogen
C
oxygen has higher boiling point than nitrogen
D
oxygen has a lower density than nitrogen
Answer :
oxygen has higher boiling point than nitrogen
443.
A water sample has ppm level concentration of following anions
$${F^ - } = 10;SO_4^{2 - } = 100;NO_3^ - = 50$$
the anion/anions that make/makes the water sample unsuitable for drinking is/are :
A
only $$NO_3^ - $$
B
both $$SO_4^{2 - }\,{\text{and}}\,NO_3^ - $$
C
only $${F^ - }$$
D
only $$SO_4^{2 - }$$
Answer :
only $${F^ - }$$
444. The symbol of element with atomic number 113, is
A
$$Nh$$
B
$$Ni$$
C
$$No$$
D
$$Nb$$
Answer :
$$Nh$$
445. A metal $$X$$ on heating in nitrogen gas gives $$Y.$$ $$Y$$ on treatment with $${H_2}O$$ gives a colourless gas which when passed through $$CuS{O_4}$$ solution gives a blue colour. $$Y$$ is
A
$$Mg{\left( {N{O_3}} \right)_2}$$
B
$$M{g_3}{N_2}$$
C
$$N{H_3}$$
D
$$MgO$$
Answer :
$$M{g_3}{N_2}$$
446.
A metal $$M$$ reacts with sodium hydroxide to give
a white precipitate $$X$$ which is soluble in excess of
$$NaOH$$ to give $$Y.$$ Compound $$X$$ is soluble in $$HCl$$ to form a compound $$Z.$$ Identify $$M, X, Y$$ and $$Z.$$
$$M$$
$$X$$
$$Y$$
$$Z$$
(a)
$$Si$$
$$Si{O_2}$$
$$N{a_2}Si{O_3}$$
$$SiC{l_4}$$
(b)
$$Al$$
$$Al{\left( {OH} \right)_3}$$
$$NaAl{O_2}$$
$$AlC{l_3}$$
(c)
$$Mg$$
$$Mg{\left( {OH} \right)_3}$$
$$NaMg{O_3}$$
$$MgC{l_2}$$
(d)
$$Ca$$
$$Ca{\left( {OH} \right)_2}$$
$$N{a_2}C{O_3}$$
$$NaHC{O_3}$$
A
(a)
B
(b)
C
(c)
D
(d)
Answer :
(b)
447. Which of the following properties correctly explain $$Si{O_2}?$$
A
Linear, basic
B
Tetrahedral, acidic
C
Tetrahedral, basic
D
Linear, acidic
Answer :
Tetrahedral, acidic
448. Which of the following elements does not show allotropy?
A
Nitrogen
B
Bismuth
C
Antimony
D
Arsenic
Answer :
Bismuth
449. Boric acid is polymeric due to
A
its acidic nature
B
the presence of hydrogen bonds
C
its monobasic nature
D
its geometry
Answer :
the presence of hydrogen bonds
450. $$Pb\,{\text{and}}\,Sn$$ are extracted from their chieforesby
A
carbon reduction and self reduction respectively
B
self reduction and carbon reduction respectively
C
electrolysis and self reduction respectively
D
self reduction and electrolysis respectively
Answer :
self reduction and carbon reduction respectively
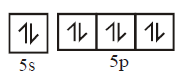
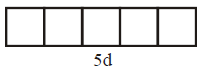 ( ground state )
( ground state )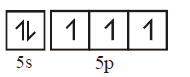
 ( third excited state )
( third excited state )