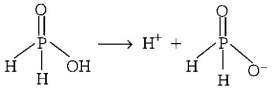161. $${H_3}P{O_2}$$ is the molecular formula of an acid of phosphorous. Its name and basicity respectively are
A
phosphorous acid and 2
B
hypophosphorous acid and 2
C
hypophosphorous acid and one
D
hypophosphoric acid and two
Answer :
hypophosphorous acid and one
162. The increasing order of atomic radii of the following Group 13 elements is
A
$$A\ell < Ga < In < T\ell $$
B
$$Ga < A\ell < In < T\ell $$
C
$$A\ell < In < Ga < T\ell $$
D
$$A\ell < Ga < T\ell < In$$
Answer :
$$Ga < A\ell < In < T\ell $$
163. The factor responsible for weak acidic nature of $$B-F$$ bonds in $$B{F_3}$$ is
A
large electronegativity of fluorine
B
three centred two electron bonds in $$B{F_3}$$
C
$$p\pi {\text{ - }}d\pi $$ back bonding
D
$$p\pi {\text{ - }}p\pi $$ back bonding
Answer :
$$p\pi {\text{ - }}p\pi $$ back bonding
164. Among the following, the correct order of acidity is
A
$$HClO < HCl{O_2} < HCl{O_3} < HCl{O_4}$$
B
$$HCl{O_2} < HClO < HCl{O_3} < HCl{O_4}$$
C
$$HCl{O_4} < HCl{O_2} < HClO < HCl{O_3}$$
D
$$HCl{O_3} < HCl{O_4} < HCl{O_2} < HClO$$
Answer :
$$HClO < HCl{O_2} < HCl{O_3} < HCl{O_4}$$
165. Electrolytic reduction of alumina to aluminium by Hall-Heroult process is carried out
A
in the presence of $$NaCl$$
B
in the presence of fluorite
C
in the presence of cryolite which forms a melt with lower melting temperature
D
in the presence of cryolite which forms a melt with higher melting temperature
Answer :
in the presence of cryolite which forms a melt with lower melting temperature
166. Excessive release of $$C{O_2}$$ into the atmosphere results in:
A
global warming
B
polar vortex
C
formation of smog
D
depletion of ozone
Answer :
global warming
167. Among $$K, Ca, Fe$$ and $$Zn,$$ the element which can form more than one binary compound with chlorine is
A
$$Fe$$
B
$$Zn$$
C
$$K$$
D
$$Ca$$
Answer :
$$Fe$$
168. Which of the following is correct representation of reaction of acidified permanganate solution with sulphur dioxide?
A
$$2MnO_4^ - + 5S{O_2} + 2{H_2}O \to $$ $$5SO_4^{2 - } + 2M{n^{2 + }} + 4{H^ + }$$
B
$$MnO_4^ - + S{O_2} + 2{H_2}O \to $$ $$S + M{n^{2 + }} + 4{H^ + }$$
C
$$2MnO_4^ - + 5S{O_2} + 2{H_2}O \to $$ $$4SO_3^{2 - } + S + 2M{n^{2 + }} + 4{H^ + }$$
D
$$2MnO_4^ - + 2S{O_2} + 2{H_2}O \to $$ $$2S + 3M{n^{2 + }} + 4{H^ + }$$
Answer :
$$2MnO_4^ - + 5S{O_2} + 2{H_2}O \to $$ $$5SO_4^{2 - } + 2M{n^{2 + }} + 4{H^ + }$$
169. The catenation tendency of $$C, Si$$ and $$Ge$$ is in the order $$Ge < Si < C.$$ The bond energies $$\left( {{\text{in}}\,kJ\,mo{l^{ - 1}}} \right)$$ of $$C - C,Si - Si$$ and $$Ge - Ge$$ bonds are respectively
A
348, 297, 260
B
297, 348, 260
C
348, 260, 297
D
260, 297, 348
Answer :
348, 297, 260
170. Shapes of certain interhalogen compounds are stated below. Which one of them is not correctly stated ?
A
$$I{F_7}:$$ pentagonal bipyramidal
B
$$Br{F_5}:$$ trigonal bipyramidal
C
$$Br{F_3}:$$ planar $$T$$-shaped
D
$$IC{I_3}:$$ planar dimeric
Answer :
$$Br{F_5}:$$ trigonal bipyramidal