191. Colour of transition metal ions are due to absorption of some wavelength. This results in
A
$$d-s$$ transition
B
$$s-s$$ transition
C
$$s-d$$ transition
D
$$d-d$$ transition
Answer :
$$d-d$$ transition
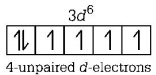
192. Which of the following has more unpaired $$d$$-electrons?
A
$$Z{n^ + }$$
B
$$F{e^{2 + }}$$
C
$${N^{3 + }}$$
D
$$C{u^ + }$$
Answer :
$$F{e^{2 + }}$$
193.
$$\eqalign{
& \left( X \right) + {K_2}C{O_3} + {\rm{Air}}\buildrel {{\rm{heat}}} \over
\longrightarrow \left( Y \right) \cr
& \left( Y \right) + C{l_2} \to \left( Z \right){\rm{Pink}} \cr} $$
Which of the following is correct ?
A
$$X = {\rm{black,}}\,Mn{O_2},\,Y = {\rm{Blue,}}\,{K_2}Cr{O_4},\,Z = KMn{O_4}$$
B
$$X = {\rm{green,}}\,C{r_2}{O_3},Y = {\rm{Yellow,}}\,{K_2}Cr{O_4},Z = {K_2}C{r_2}{O_7}$$
C
$$X = {\rm{black,}}\,Mn{O_2},Y = {\rm{green,}}\,{K_2}Mn{O_4},Z = KMn{O_4}$$
D
$$X = {\rm{black,}}\,B{i_2}{O_3},\,Y = {\rm{colourless}}\,\,KBi{O_2},Z = KBi{O_3}$$
Answer :
$$X = {\rm{black,}}\,Mn{O_2},Y = {\rm{green,}}\,{K_2}Mn{O_4},Z = KMn{O_4}$$
194. When same amount of zinc is treated separately with excess of sulphuric acid and excess of sodium hydroxide, the ratio of volume of hydrogen evolved is
A
$$1:1$$
B
$$1:2$$
C
$$2:1$$
D
$$9:4$$
Answer :
$$1:1$$
195. In context of the lanthanoids, which of the following statements is not correct?
A
There is a gradual decrease in the radii of the members with increasing atomic number in the series.
B
All the members exhibit + 3 oxidation state.
C
Because of similar properties the separation of lanthanoids is not easy.
D
Availability of $$4f$$ electrons results in the formation of compounds in + 4 state for all the members of the series.
Answer :
Availability of $$4f$$ electrons results in the formation of compounds in + 4 state for all the members of the series.
196. Among the properties (a) reducing (b) oxidising (c) complexing, the set of properties shown by $$C{N^ - }\,{\text{ion}}$$ towards metal species is
A
$${\text{c,a}}$$
B
$${\text{b,c}}$$
C
$${\text{a,b}}$$
D
$${\text{a,b,c}}$$
Answer :
$${\text{c,a}}$$
197. Identify the species in which the metal atom is in +6 oxidation state.
A
$$MnO_4^ - $$
B
$${\left[ {Cr{{\left( {CN} \right)}_6}} \right]^{3 - }}$$
C
$${\left[ {Ni{F_6}} \right]^{2 - }}$$
D
$$Cr{O_2}C{l_2}$$
Answer :
$$Cr{O_2}C{l_2}$$
198. The transition elements have a general electronic configuration.
A
$$n{s^2}n{p^6}n{d^{1 - 10}}$$
B
$$\left( {n - 1} \right){d^{1 - 10}},n{s^{0 - 2}},n{p^{0 - 6}}$$
C
$$\left( {n - 1} \right){d^{1 - 10}},n{s^{1 - 2}}$$
D
$$n{d^{1 - 10}}n{s^2}$$
Answer :
$$\left( {n - 1} \right){d^{1 - 10}},n{s^{1 - 2}}$$
199.
Which of the following reactions are involved in the process of preparation of potassium dichromate?
$$\eqalign{
& \left( {\text{i}} \right)4FeC{r_2}{O_4} + 8N{a_2}C{O_3} + 7{O_2} \to \cr
& \left( {{\text{ii}}} \right)N{a_2}Cr{O_4} + {H_2}S{O_4} \to \cr
& \left( {{\text{iii}}} \right)N{a_2}C{r_2}{O_7} + 2KCl \to \cr} $$
A
(i) and (ii)
B
(ii) and (iii)
C
(i) and (iii)
D
(i), (ii) and (iii)
Answer :
(i), (ii) and (iii)
200. Which of the following have maximum and minimum ionic character out of $$MnO,Mn{F_2},Mn{O_2},M{n_2}{O_7}?$$
A
$$MnO$$ and $$M{n_2}{O_7}$$ respectively
B
$$Mn{F_2}$$ and $$M{n_2}{O_7}$$ respectively
C
$$Mn{O_2}$$ and $$MnO$$ respectively
D
$$M{n_2}{O_7}$$ and $$MnO$$ respectively
Answer :
$$Mn{F_2}$$ and $$M{n_2}{O_7}$$ respectively