81. $$C{N^ - }$$ is strong field ligand. This is due to the fact that
A
it carries negative charge
B
it is a pseudohalide
C
it can accept electrons from metal species
D
it forms high spin complexes with metal species
Answer :
it is a pseudohalide
82.
Match the column I with column II and mark the appropriate choice.
Column I
Column II
a.
$${\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{3 - }}$$
1.
$$0\,B.M.$$
b.
$${\left[ {Co{F_6}} \right]^{3 - }}$$
2.
$$5.92\,B.M.$$
c.
$${\left[ {Fe{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }}$$
3.
$$4.89\,B.M.$$
d.
$${\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }}$$
4.
$$1.732\,B.M.$$
A
a - 2, b - 3, c - 4, d - 1
B
a - 3, b - 2, c - 1, d - 4
C
a - 1, b - 3, c - 4, d - 2
D
a - 4, b - 3, c - 2, d - 1
Answer :
a - 4, b - 3, c - 2, d - 1
83. Which of the following complex species is not expected to exhibit optical isomerism?
A
$${\left[ {Co\left( {en} \right){{\left( {N{H_3}} \right)}_2}C{l_2}} \right]^ + }$$
B
$${\left[ {Co{{\left( {en} \right)}_3}} \right]^{3 + }}$$
C
$${\left[ {Co{{\left( {en} \right)}_2}C{l_2}} \right]^ + }$$
D
$$\left[ {Co{{\left( {N{H_3}} \right)}_3}C{l_3}} \right]$$
Answer :
$$\left[ {Co{{\left( {N{H_3}} \right)}_3}C{l_3}} \right]$$
84. The correct IUPAC name of the compound $$\left[ {Cr{{\left( {N{H_3}} \right)}_5}\left( {NCS} \right)} \right]\left[ {ZnC{l_4}} \right]$$ is
A
pentaammineisothiocyanidochromium(III) tetrachloridozincate(II)
B
pentammineisothiocyanidozinc chloridechromate(III)
C
pentaammineisothiocyanidochromate(II) zinc chloride(IV)
D
isothiocyanidopentaamminechromium(II) zinc chloride(IV)
Answer :
pentaammineisothiocyanidochromium(III) tetrachloridozincate(II)
85.
Match column-I with column-II and select the correct answer.
$$\left\{ {en = {H_2}NC{H_2}C{H_2}N{H_2};{\text{At}}{\text{.}}\,{\text{Nos}}{\text{.}}:Ti = 22;Cr = 24;Co = 27;Pt = 78} \right\}$$
Column I
Column II
(P)
$$\left[ {Cr{{\left( {N{H_3}} \right)}_4}C{l_2}} \right]Cl$$
1.
Paramagnetic and exhibits ionisation isomerism
(Q)
$$\left[ {Ti{{\left( {{H_2}O} \right)}_5}Cl} \right]{\left. {N{O_3}} \right)_2}$$
2.
Diamagnetic and exhibits $$cis - trans$$ isomerism
(R)
$$\left[ {Pt\left( {en} \right)\left( {N{H_3}} \right)Cl} \right]N{O_3}$$
3.
Paramagnetic and exhibits $$cis - trans$$ isomerism
(S)
$$\left[ {Co{{\left( {N{H_3}} \right)}_4}{{\left( {N{O_3}} \right)}_2}} \right]N{O_3}$$
4.
Diamagnetic and exhibits ionisation isomerism
A
P - 4, Q - 2, R - 3, S - 1
B
P - 3, Q - 1, R - 4, S - 2
C
P - 2, Q - 1, R - 3, S - 4
D
P -1 , Q - 3, R - 4, S - 2
Answer :
P - 3, Q - 1, R - 4, S - 2
86. In which of the following coordinate compounds the central metal atom obeys the $$EAN$$ rule.
A
$${K_3}\left[ {Fe{{\left( {CN} \right)}_6}} \right]$$
B
$${K_4}\left[ {Fe{{\left( {CN} \right)}_6}} \right]$$
C
$$\left[ {Cu{{\left( {N{H_3}} \right)}_4}} \right]S{O_4}$$
D
$${\text{All of these}}$$
Answer :
$${K_4}\left[ {Fe{{\left( {CN} \right)}_6}} \right]$$
87. Which of the following complex ions is not expected to absorb visible light ?
A
$${\left[ {Ni{{\left( {CN} \right)}_4}} \right]^{2 - }}$$
B
$${\left[ {Cr{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }}$$
C
$${\left[ {Fe{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
D
$${\left[ {Ni{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
Answer :
$${\left[ {Ni{{\left( {CN} \right)}_4}} \right]^{2 - }}$$
88.
Among the following complexes $$(K-P)$$
$${K_3}\left[ {Fe{{\left( {CN} \right)}_6}} \right]\left( K \right),$$ $$\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]C{l_3}\left( L \right),$$ $$N{a_3}\left[ {Co{{{\text{(oxalate)}}}_3}} \right]\left( M \right),$$ $$\left[ {Ni{{\left( {{H_2}O} \right)}_6}} \right]C{l_2}\left( N \right),$$ $${K_2}\left[ {Pt{{\left( {CN} \right)}_4}} \right]\left( O \right)$$ and $$\left[ {Zn{{\left( {{H_2}O} \right)}_6}} \right]{\left( {N{O_3}} \right)_2}\left( P \right)$$
A
$$K, L, M, N$$
B
$$K, M, O, P$$
C
$$L, M, O, P$$
D
$$L, M, N, O$$
Answer :
$$L, M, O, P$$
89.
Arrange the following complexes in increasing order of conductivity of their solutions.
$$\eqalign{
& \left( {\text{i}} \right)\left[ {Co{{\left( {N{H_3}} \right)}_3}C{l_3}} \right] \cr
& \left( {{\text{ii}}} \right)\left[ {Co{{\left( {N{H_3}} \right)}_4}C{l_2}} \right]Cl \cr
& \left( {{\text{iii}}} \right)\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]C{l_3} \cr
& \left( {{\text{iv}}} \right)\left[ {Co{{\left( {N{H_3}} \right)}_5}Cl} \right]C{l_2} \cr} $$
A
(i) < (ii) < (iv) < (iii)
B
(ii) < (i) < (iii) < (iv)
C
(i) < (iii) < (ii) < (iv)
D
(iv) < (i) < (ii) < (iii)
Answer :
(i) < (ii) < (iv) < (iii)
90. IUPAC name of $$\left[ {Pt{{\left( {N{H_3}} \right)}_3}\left( {Br} \right)\left( {N{O_2}} \right)Cl} \right]Cl$$ is
A
triamminebromochloronitroplatinum (IV) chloride
B
triamminebromonitrochloroplatinum (IV) chloride
C
triamminechlorobromonitroplatinum (IV) chloride
D
triamminenitrochlorobromoplatinum (IV) chloride
Answer :
triamminebromochloronitroplatinum (IV) chloride
 $$\left[ {\because n = 1} \right]$$
$$\left[ {\because n = 1} \right]$$ $$\left[ {\because n = 4} \right]$$
$$\left[ {\because n = 4} \right]$$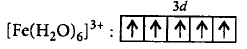 $$\left[ {\because n = 5} \right]$$
$$\left[ {\because n = 5} \right]$$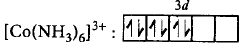 $$\left[ {\because n = 0} \right]$$
$$\left[ {\because n = 0} \right]$$
