61. Which statement is incorrect ?
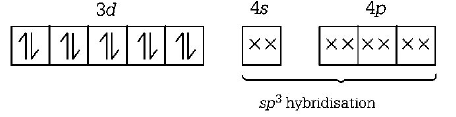
A
$$Ni{\left( {CO} \right)_4}{\text{ - }}$$ tetrahedral, paramagnetic
B
$${\left[ {Ni{{\left( {CN} \right)}_4}} \right]^{2 - }}{\text{ - }}$$ square planar, diamagnetic
C
$$Ni{\left( {CO} \right)_4}{\text{ - }}$$ tetrahedral, diamagnetic
D
$${\left[ {Ni{{\left( {Cl} \right)}_4}} \right]^{2 - }}$$ tetrahedral, paramagnetic
Answer :
$$Ni{\left( {CO} \right)_4}{\text{ - }}$$ tetrahedral, paramagnetic
62. Among the following species the one which causes the highest $$CFSE,{\Delta _ \circ }$$ as a ligand is :
A
$$C{N^ - }$$
B
$$N{H_3}$$
C
$${F^ - }$$
D
$$CO$$
Answer :
$$CO$$
63. Tetraammineaquachloridocobalt (III) chloride can be written as
A
$$\left[ {CoCl\left( {{H_2}O} \right){{\left( {N{H_3}} \right)}_4}} \right]Cl$$
B
$$\left[ {CoCl\left( {{H_2}O} \right){{\left( {N{H_3}} \right)}_4}} \right]C{l_2}$$
C
$$\left[ {CoCl\left( {{H_2}O} \right){{\left( {N{H_3}} \right)}_4}Cl} \right]$$
D
$${\text{none of these}}$$
Answer :
$$\left[ {CoCl\left( {{H_2}O} \right){{\left( {N{H_3}} \right)}_4}} \right]C{l_2}$$
64. Which of the following complexes have a maximum number of unpaired electrons?
A
$$\left[ {Ni{{\left( {CO} \right)}_4}} \right]$$
B
$${\left[ {Co{{\left( {N{H_3}} \right)}_4}{{\left( {N{O_2}} \right)}_2}} \right]^ + }$$
C
$${\left[ {Ag{{\left( {CN} \right)}_2}} \right]^ - }$$
D
$${\left[ {CuB{r_4}} \right]^{2 - }}$$
Answer :
$${\left[ {CuB{r_4}} \right]^{2 - }}$$
65. What kind of isomerism exists between $$\left[ {Cr{{\left( {{H_2}O} \right)}_6}} \right]C{l_3}$$ (violet) and $$\left[ {Cr{{\left( {{H_2}O} \right)}_5}Cl} \right]C{l_2} \cdot {H_2}O$$ ( greyish - green )?
A
Linkage isomerism
B
Solvate isomerism
C
Ionisation isomerism
D
Coordination isomerism
Answer :
Solvate isomerism
66. Which complex of $$C{o^{2 + }}$$ will have the weakest crystal field splitting –
A
$${\left[ {CoC{l_6}} \right]^{4 - }}$$
B
$${\left[ {Co{{\left( {CN} \right)}_6}} \right]^{4 - }}$$
C
$${\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]^{2 + }}$$
D
$${\left[ {Co{{\left( {en} \right)}_3}} \right]^{2 + }}$$
Answer :
$${\left[ {CoC{l_6}} \right]^{4 - }}$$
67. Square-planar geometry is shown by
A
$$\left[ {PtC{l_2}{{\left( {N{H_3}} \right)}_2}} \right]$$
B
$${\left[ {NiC{l_4}} \right]^{2 - }}$$
C
$$MnO_4^ - $$
D
$$CrO_4^{2 - }$$
Answer :
$$\left[ {PtC{l_2}{{\left( {N{H_3}} \right)}_2}} \right]$$
68.
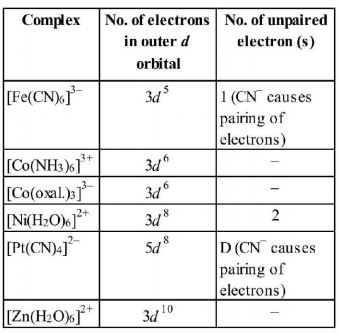
Among the following complexes $$(K-P)$$
$${K_3}\left[ {Fe{{\left( {CN} \right)}_6}} \right]\left( K \right),$$ $$\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]C{l_3}\left( L \right),$$ $$N{a_3}\left[ {Co{{\left( {{\text{oxalate}}} \right)}_3}} \right]\left( M \right),$$ the $$\left[ {Ni{{\left( {{H_2}O} \right)}_6}} \right]C{l_2}\left( N \right),$$ $${K_2}\left[ {Pt{{\left( {CN} \right)}_4}} \right]\left( O \right)$$ and $$\left[ {Zn{{\left( {{H_2}O} \right)}_6}} \right]{\left( {N{O_3}} \right)_2}\left( P \right)$$ the diamagnetic complexes are
A
$$K,L,M,N$$
B
$$K,M,O,P $$
C
$$L,M,O,P $$
D
$$L,M,N,O $$
Answer :
$$L,M,O,P $$
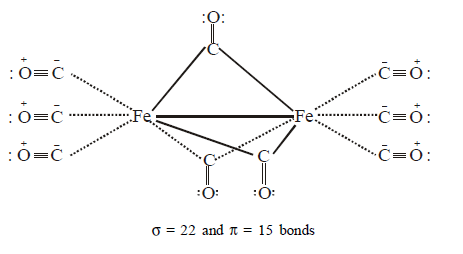
69. The number of $$\sigma $$ and $$\pi $$ bonds in $$F{e_2}{\left( {CO} \right)_9},$$ respectively, are
A
$$22\sigma \,{\text{and}}\,15\pi $$
B
$$22\sigma \,{\text{and}}\,16\pi $$
C
$$23\sigma \,{\text{and}}\,15\pi $$
D
$$15\sigma \,{\text{and}}\,8\pi $$
Answer :
$$22\sigma \,{\text{and}}\,15\pi $$
70. $$\left[ {Pt{{\left( {N{H_3}} \right)}_4}} \right]\left[ {CuC{l_4}} \right]$$ and $$\left[ {Cu{{\left( {N{H_3}} \right)}_4}} \right]\left[ {PtC{l_4}} \right]$$ are known as
A
ionisation isomers
B
coordination isomers
C
linkage isomers
D
polymerisation isomers
Answer :
coordination isomers