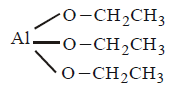181. Coordination number of $$Ni$$ in $${\left[ {Ni{{\left( {{C_2}{O_4}} \right)}_3}} \right]^{4 - }}$$ is
A
3
B
6
C
4
D
2
Answer :
6
182. Which of the following does not have a metalcarbon bond ?
A
$$Al{\left( {O{C_2}{H_5}} \right)_3}$$
B
$${C_2}{H_5}MgBr$$
C
$$K\left[ {Pt\left( {{C_2}{H_4}} \right)C{l_3}} \right]$$
D
$$Ni{\left( {CO} \right)_4}$$
Answer :
$$Al{\left( {O{C_2}{H_5}} \right)_3}$$
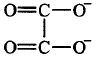
183. Which of the following ligands form a chelate?
A
Acetate
B
Oxalate
C
Cyanide
D
Ammonia
Answer :
Oxalate
184. Complexes $$\left[ {Co{{\left( {N{H_3}} \right)}_5}S{O_4}} \right]Br$$ and $$\left[ {Co{{\left( {N{H_3}} \right)}_5}Br} \right]S{O_4}$$ can be distinguished by
A
conductance measurement
B
using $$BaC{l_2}$$
C
using $$AgN{O_3}$$
D
Both (B) and (C)
Answer :
Both (B) and (C)
185. Which one of the following statements is not correct ?
A
Mercury (II) iodide dissolves in excess of potassium iodide solution
B
Tin (IV) chloride is made by dissolving tin solution in concentrated hydrochloric acid
C
Zinc dissolves in sodium hydroxide solution
D
Carbon monoxide reduces iron (III) oxide to iron
Answer :
Tin (IV) chloride is made by dissolving tin solution in concentrated hydrochloric acid
186. Which kind of isomerism is exhibited by octahedral $$Co{\left( {N{H_3}} \right)_4}B{r_2}Cl$$ ?
A
Geometrical and Ionization
B
Geometrical and Optical
C
Optical and Ionization
D
Geometrical only
Answer :
Geometrical and Ionization
187. The colour of the coordination compounds depends on the crystal field splitting. What will be the correct order of absorption of wavelength of light in the visible region, for the complexes, $${\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }},{\left[ {Co{{\left( {CN} \right)}_6}} \right]^{3 - }},$$ $${\left[ {Co{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }}?$$
A
$${\left[ {Co{{\left( {CN} \right)}_6}} \right]^{3 - }} > {\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }}$$ $$ > {\left[ {Co{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }}$$
B
$${\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }} > {\left[ {Co{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }}$$ $$ > {\left[ {Co{{\left( {CN} \right)}_6}} \right]^{3 - }}$$
C
$${\left[ {Co{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }} > {\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }}$$ $$ > {\left[ {Co{{\left( {CN} \right)}_6}} \right]^{3 - }}$$
D
$${\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }} > {\left[ {Co{{\left( {CN} \right)}_6}} \right]^{3 - }}$$ $$ > {\left[ {Co{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }}$$
Answer :
$${\left[ {Co{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }} > {\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }}$$ $$ > {\left[ {Co{{\left( {CN} \right)}_6}} \right]^{3 - }}$$
188. The formula of dichlorobis (urea) copper (II) is
A
$$\left[ {Cu\left\{ {O = C{{\left( {N{H_2}} \right)}_2}Cl} \right\}} \right]Cl$$
B
$$\left[ {CuC{l_2}{{\left\{ {O = C{{\left( {N{H_2}} \right)}_2}} \right\}}_2}} \right]$$
C
$$\left[ {Cu\left\{ {O = C{{\left( {N{H_2}} \right)}_2}} \right\}} \right]C{l_2}$$
D
$$\left[ {CuC{l_2}\left\{ {O = C{{\left( {N{H_2}} \right)}_2}{H_2}} \right\}} \right]$$
Answer :
$$\left[ {CuC{l_2}{{\left\{ {O = C{{\left( {N{H_2}} \right)}_2}} \right\}}_2}} \right]$$
189. Which of the following primary and secondary valencies are not correctly marked against the compound?
A
$$\left[ {Cr{{\left( {N{H_3}} \right)}_6}} \right]C{l_3},p = 3,s = 6$$
B
$${K_2}\left[ {PtC{l_4}} \right],p = 2,s = 4$$
C
$$\left[ {Pt{{\left( {N{H_3}} \right)}_2}C{l_2}} \right],p = 2,s = 4$$
D
$$\left[ {Cu{{\left( {N{H_3}} \right)}_4}} \right]S{O_4},p = 4,s = 4$$
Answer :
$$\left[ {Cu{{\left( {N{H_3}} \right)}_4}} \right]S{O_4},p = 4,s = 4$$
190. A compound contains $$1.08\,mole$$ of $$Na,0.539\,mole$$ of $$Cu$$ and $$2.16\,mole$$ of $$F.$$ Its aqueous solution shows osmotic pressure which is three times that of urea having same molar concentration. The formula of the compound is :
A
$$N{a_4}\left[ {Cu{F_6}} \right]$$
B
$$Na\left[ {Cu{F_4}} \right]$$
C
$$N{a_2}\left[ {Cu{F_4}} \right]$$
D
$$N{a_2}\left[ {Cu{F_3}} \right]$$
Answer :
$$N{a_2}\left[ {Cu{F_4}} \right]$$