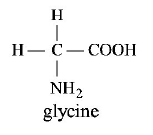91. Dinucleotide is obtained by joining two nucleotides together by phosphodiester linkage. Between which carbon atoms of pentose sugars of nucleotides are these linkages present?
A
5' and 3'
B
1' and 5'
C
5' and 5'
D
3' and 3'
Answer :
5' and 3'
92. Amino acids are least soluble
A
at $$pH$$ around $$7$$
B
at $$pH\,7$$
C
at their isoelectric points
D
none of these
Answer :
at their isoelectric points
93. Glycolysis is
A
oxidation of glucose to pyruvate
B
conversion of glucose to haem
C
oxidation of glucose to glutarnate
D
conversion of pyruvate to citrate
Answer :
oxidation of glucose to pyruvate
94. Which functional group participates in disulphide bond formation in proteins?
A
Thiolactone
B
Thiol
C
Thioether
D
Thioester
Answer :
Thiol
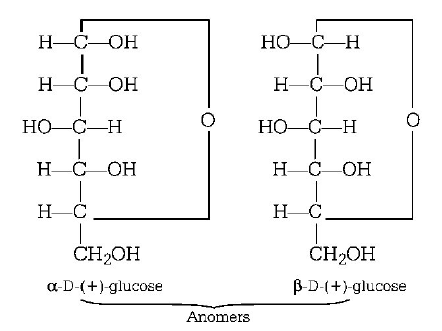
95. $$\alpha {\text{ - }}D{\text{ - }}\left( + \right){\text{ - }}$$ glucose and $$\beta {\text{ - }}D{\text{ - }}\left( + \right){\text{ - }}$$ glucose are
A
anomers
B
epimers
C
enantiomers
D
geometrical isomers
Answer :
anomers
96. Which one of the following statements is correct?
A
All amino acids except lysine are optically active
B
All amino acids are optically active
C
All amino acids except glycine are optically active
D
All amino acids except glutamic acids are optically
active
Answer :
All amino acids except glycine are optically active
97. Enzymes are made up of
A
edible proteins
B
proteins with specific structure
C
nitrogen containing carbohydrates
D
Carbohydrates
Answer :
proteins with specific structure
98. Which of the following is basic amino acid?
A
Lysine
B
Proline
C
Alanine
D
Aspartic acid
Answer :
Lysine
99. Which of the following is responsible for preparing the uterus for implantation of fertilised egg?
A
Testosterone
B
Glucocorticoids
C
Progesterone
D
Estradiol
Answer :
Progesterone
100. Glutamic acid, $${H_2}N - CH\left( {C{H_2}C{H_2}COOH} \right)$$ $$.COOH$$ has $$p{K_{{a_1}}},\left( {\alpha - COOH} \right) = 2.2,$$ $$p{K_{{a_2}}}\left( {\alpha - N{H_3}} \right) = 9.8$$ and $$p{K_{{a_3}}}\left( {R\,\,{\text{group}}\,\,COOH} \right) = 4.3.$$ The isoelectric point of glutamic acid is
A
5.9
B
7
C
10.25
D
3.25
Answer :
3.25