Question
$$X$$ and $$Y$$ are two volatile liquids with molar weights of $$10\,g\,mo{l^{ - 1}}$$ and $$40\,g\,mo{l^{ - 1}}$$ respectively. Two cotton plugs, one soaked in $$X$$ and the other soaked in $$Y,$$ are Simultaneously placed at the ends of a tube of length $$L = 24\,cm,$$ as shown in the figure. The tube is filled with an inert gas at 1atmosphere pressure and a temperature of $$300\,K.$$ Vapours of $$X$$ and $$Y$$ react to form a product which is first observed at a distance $$d\,cm$$ from the plug soaked in $$X.$$ Take $$X$$ and $$Y$$ to have equal molecular diameters and assume ideal behaviour for the inert gas and the vapours.
$$X$$ and $$Y$$ are two volatile liquids with molar weights of $$10\,g\,mo{l^{ - 1}}$$ and $$40\,g\,mo{l^{ - 1}}$$ respectively. Two cotton plugs, one soaked in $$X$$ and the other soaked in $$Y,$$ are Simultaneously placed at the ends of a tube of length $$L = 24\,cm,$$ as shown in the figure. The tube is filled with an inert gas at 1atmosphere pressure and a temperature of $$300\,K.$$ Vapours of $$X$$ and $$Y$$ react to form a product which is first observed at a distance $$d\,cm$$ from the plug soaked in $$X.$$ Take $$X$$ and $$Y$$ to have equal molecular diameters and assume ideal behaviour for the inert gas and the vapours.
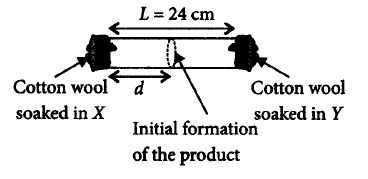
The value of $$d$$ in $$cm$$ ( shown in the figure ), as estimated from Graham's law, is
A.
8
B.
12
C.
16
D.
20
Answer :
16
Solution :
According to Graham's law, $$r \propto \frac{1}{{\sqrt M }}$$
As all conditions are identical for $$X$$ and $$Y,$$
$$\eqalign{ & \frac{{{r_X}}}{{{r_Y}}} = \sqrt {\frac{{{M_Y}}}{{{M_X}}}} \Rightarrow \frac{d}{{24 - d}} = \sqrt {\frac{{40}}{{10}}} = 2 \cr & d = 48 - 2d \Rightarrow 3d = 48 \Rightarrow d = 16\,cm \cr} $$
According to Graham's law, $$r \propto \frac{1}{{\sqrt M }}$$
As all conditions are identical for $$X$$ and $$Y,$$
$$\eqalign{ & \frac{{{r_X}}}{{{r_Y}}} = \sqrt {\frac{{{M_Y}}}{{{M_X}}}} \Rightarrow \frac{d}{{24 - d}} = \sqrt {\frac{{40}}{{10}}} = 2 \cr & d = 48 - 2d \Rightarrow 3d = 48 \Rightarrow d = 16\,cm \cr} $$