Question
Which one of the following substituents at $$para$$ - position is most effective in stabilizing the phenoxide
Which one of the following substituents at $$para$$ - position is most effective in stabilizing the phenoxide 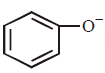 ion ?
ion ?
A.
$$ - C{H_3}$$
B.
$$ - OC{H_3}$$
C.
$$ - COC{H_3}$$
D.
$$ - C{H_2}OH$$
Answer :
$$ - COC{H_3}$$
Solution :
Electron withdrawing group stabilises the benzene ring due to delocalisation of charge.
$$ - C{H_3}$$ and $$ - C{H_2}OH$$ are electron donating group and hence decrease the stability of benzene ring $$ - OC{H_3}$$ is weaker electron withdrawing group than $$ - COC{H_3}.$$ Hence $$ - COC{H_3}$$ group more stabilize the phenoxide ion at $$p$$ - position.
Electron withdrawing group stabilises the benzene ring due to delocalisation of charge.
$$ - C{H_3}$$ and $$ - C{H_2}OH$$ are electron donating group and hence decrease the stability of benzene ring $$ - OC{H_3}$$ is weaker electron withdrawing group than $$ - COC{H_3}.$$ Hence $$ - COC{H_3}$$ group more stabilize the phenoxide ion at $$p$$ - position.