Question
Which one of the following orders of acidic strength is correct ?
A.
$$RCOOH > HOH > HC \equiv CH > ROH$$
B.
$$RCOOH > HC \equiv CH > HOH > ROH$$
C.
$$RCOOH > ROH > HOH > HC \equiv CH$$
D.
$$RCOOH > HOH > ROH > HC \equiv CH$$
Answer :
$$RCOOH > HOH > ROH > HC \equiv CH$$
Solution :
Carboxylic acid is stronger than alcohol and water because after removal of proton, carboxylate ion is stabilised by resonance. Hence, correct order of acid strength is
$$RCOOH > HOH > ROH > HC \equiv CH$$
Which is based upon the rate of donation of proton or strength of base, thus order of basic strength is $$\mathop C\limits^ - \equiv CH > R - \mathop O\limits^ - > O{H^ - } > RCO{O^ - }$$
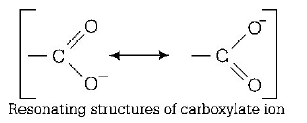
Carboxylic acid is stronger than alcohol and water because after removal of proton, carboxylate ion is stabilised by resonance. Hence, correct order of acid strength is
$$RCOOH > HOH > ROH > HC \equiv CH$$
Which is based upon the rate of donation of proton or strength of base, thus order of basic strength is $$\mathop C\limits^ - \equiv CH > R - \mathop O\limits^ - > O{H^ - } > RCO{O^ - }$$
