Question
Which one of the following is most reactive towards electrophilic attack?
A.
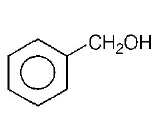

B.


C.
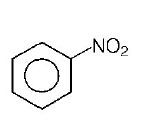

D.
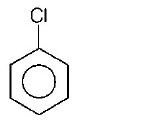

Answer :


Solution :
The group showing electron-donating effect ( such as $$ - N{H_2}, - OH$$ ) should stabilise the intermediate $$ions,$$ i.e. makes the ring more reactive towards electrophilic substitution than benzene and are called activating group while electron withdrawing group ( such as $$ - Cl,N{O_2}$$ ) increases the positive charge on ring, thus deactivates the ring. Hence, phenol is more readily attacked by an electrophile and is most reactive towards an electroptile .
The group showing electron-donating effect ( such as $$ - N{H_2}, - OH$$ ) should stabilise the intermediate $$ions,$$ i.e. makes the ring more reactive towards electrophilic substitution than benzene and are called activating group while electron withdrawing group ( such as $$ - Cl,N{O_2}$$ ) increases the positive charge on ring, thus deactivates the ring. Hence, phenol is more readily attacked by an electrophile and is most reactive towards an electroptile .