Question
Which one of the following formulae does not correctly represent the bonding capacities of the atoms involved?
A.
.PNG)
.PNG)
B.
.PNG)
.PNG)
C.
.PNG)
.PNG)
D.
.PNG)
.PNG)
Answer :
.PNG)
.PNG)
Solution :
In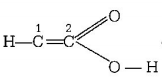 carbon number $$2$$ have five valency which is not possible, so it does not correctly represent the bonding capacities of $$C$$ atom.
carbon number $$2$$ have five valency which is not possible, so it does not correctly represent the bonding capacities of $$C$$ atom.
In
 carbon number $$2$$ have five valency which is not possible, so it does not correctly represent the bonding capacities of $$C$$ atom.
carbon number $$2$$ have five valency which is not possible, so it does not correctly represent the bonding capacities of $$C$$ atom.