Question
Which one of the following esters gets hydrolysed most easily under alkaline conditions?
A.


B.
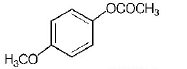

C.


D.


Answer :


Solution :
Electron withdrawing group attach to the benzene ring increases the reactivity towards nucleophilic sustitution reaction. Since $$ - N{O_2}$$ group is strong electron withdrawing group. Hence in basic medium ester containing $$ - N{O_2}$$ group Will hydrolysed most easily.
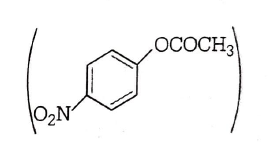
Electron withdrawing group attach to the benzene ring increases the reactivity towards nucleophilic sustitution reaction. Since $$ - N{O_2}$$ group is strong electron withdrawing group. Hence in basic medium ester containing $$ - N{O_2}$$ group Will hydrolysed most easily.
