Question
Which one of the following compounds will be most easily attacked by an electrophile?
A.


B.


C.
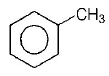

D.
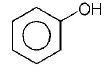

Answer :


Solution :
$$ - Cl{\text{ - }}atom$$ shows $$+R$$ - effect that $$o/p$$ -directive influence but deactivate the benzene ring. While $$ - OH, - C{H_3}$$ group also shows $$o/p$$ -influence but activate the benzene ring. But in these $$-OH$$ group activates more than $$ - C{H_3}.$$
Hence, order of electrophilic substitution is
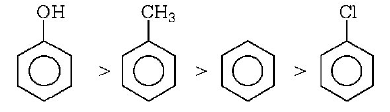
$$ - Cl{\text{ - }}atom$$ shows $$+R$$ - effect that $$o/p$$ -directive influence but deactivate the benzene ring. While $$ - OH, - C{H_3}$$ group also shows $$o/p$$ -influence but activate the benzene ring. But in these $$-OH$$ group activates more than $$ - C{H_3}.$$
Hence, order of electrophilic substitution is
