Question
Which one of the following compounds has the most acidic nature?
A.


B.
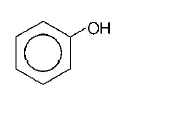

C.
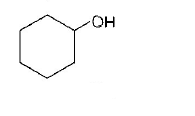

D.
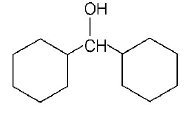

Answer :


Solution :
Key Idea Presence of electron withdrawing substituent increases the acidity while electron releasing substituent, decreases the acidity.
Phenyl is an electron withdrawing substituent while $$ - C{H_3}$$ is an electron releasing substituent. Moreover, phenoxide ion is more resonance stabilised as compared to benzyloxide ion, thus releases proton more easily. That’s why phenol is a strong acid among the given compounds.
Key Idea Presence of electron withdrawing substituent increases the acidity while electron releasing substituent, decreases the acidity.
Phenyl is an electron withdrawing substituent while $$ - C{H_3}$$ is an electron releasing substituent. Moreover, phenoxide ion is more resonance stabilised as compared to benzyloxide ion, thus releases proton more easily. That’s why phenol is a strong acid among the given compounds.