Question
Which of the following would react most readily with nucleophiles ?
A.
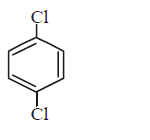

B.
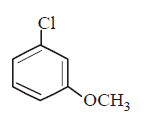

C.


D.
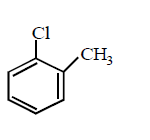

Answer :


Solution :
Aryl halides do not undergo nucleophilic substitution under ordinary conditions. The low reactivity of halogen atom in aryl halides is due to resonance. However, aryl halides can be made to undergo nucleophilic substitution either under drastic condition ( high temperature, pressure or very strong nucleophiles ) or by activating the nuclear halogen by introducing electron withdrawing group e.g. $$N{O_2}, - CHO,CN$$ etc. in the $$o - $$ and $$p - $$ position to the nuclear halogen. Hence, $$p - $$ nitrochlorobenzene would react most readily with nucleophiles.
Aryl halides do not undergo nucleophilic substitution under ordinary conditions. The low reactivity of halogen atom in aryl halides is due to resonance. However, aryl halides can be made to undergo nucleophilic substitution either under drastic condition ( high temperature, pressure or very strong nucleophiles ) or by activating the nuclear halogen by introducing electron withdrawing group e.g. $$N{O_2}, - CHO,CN$$ etc. in the $$o - $$ and $$p - $$ position to the nuclear halogen. Hence, $$p - $$ nitrochlorobenzene would react most readily with nucleophiles.