Question
Which of the following steps is not correct in the mechanism of electrophilic substitution of benzene?
A.
Generation of electrophile like $${X^ + },{R^ + },NO_2^ + ,$$ etc.
B.
Attack of electrophile resulting in the formation of arenium ion in which one of the carbon is $$s{p^3}$$ hybridised.
C.
Addition of proton on benzene ring to give carbocation.
D.
Removal of proton from $$s{p^3}$$ carbon atom to restore aromatic character.
Answer :
Addition of proton on benzene ring to give carbocation.
Solution :
Arenium ion is formed as intermediate by the attack of electrophile not by addition of proton. Proton is substituted by electrophile.
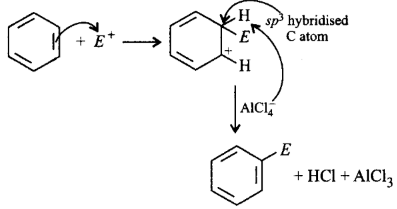
Arenium ion is formed as intermediate by the attack of electrophile not by addition of proton. Proton is substituted by electrophile.
