Question
Which of the following statements is correct regarding the structure of $$PC{l_5}?$$
A.
Three $$P - Cl$$ bonds lie in one plane and two $$P - Cl$$ bonds lie above and below the equatorial plane.
B.
Five $$P - Cl$$ bonds lie in the same plane.
C.
The bond angle in all $$P - Cl$$ bonds is $${90^ \circ }.$$
D.
The bond length of all $$P - Cl$$ bonds is same.
Answer :
Three $$P - Cl$$ bonds lie in one plane and two $$P - Cl$$ bonds lie above and below the equatorial plane.
Solution :
In $$PC{l_5},$$ two axial and three equatorial bonds are present.
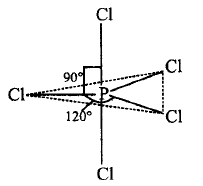
The bond length of all $$P - Cl$$ bonds are not same.
In $$PC{l_5},$$ two axial and three equatorial bonds are present.

The bond length of all $$P - Cl$$ bonds are not same.