Question
Which of the following species has a linear shape?
A.
$$NO_2^ - $$
B.
$$S{O_2}$$
C.
$$NO_2^ + $$
D.
$${O_3}$$
Answer :
$$NO_2^ + $$
Solution :
$$\mathop N\limits^ + {O_2}$$ has linear shape due to $$sp$$ hybridisation of $$N$$ in $$\mathop N\limits^ + {O_2}$$
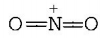
While $$S{O_2},NO_2^ - $$ and $${O_3}$$ have angular shape
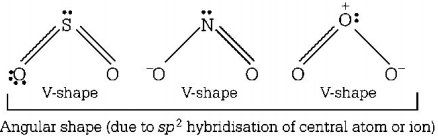
$$\mathop N\limits^ + {O_2}$$ has linear shape due to $$sp$$ hybridisation of $$N$$ in $$\mathop N\limits^ + {O_2}$$
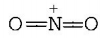
While $$S{O_2},NO_2^ - $$ and $${O_3}$$ have angular shape
