Question
Which of the following species contains three bond pairs and one lone pair around the central atom?
A.
$${H_2}O$$
B.
$$B{F_3}$$
C.
$$NH_2^ - $$
D.
$$PC{l_3}$$
Answer :
$$PC{l_3}$$
Solution :
$$\left( {\text{A}} \right){H_2}O \Rightarrow $$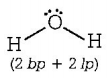
$$\left[ {bp = {\text{bond}}\,{\text{pair}}\,{\text{and}}\,lp = {\text{lone}}\,{\text{pair}}} \right]$$
$$\left( {\text{B}} \right)B{F_3} \Rightarrow $$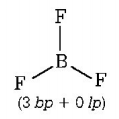
$$\left( {\text{C}} \right)NH_2^ - \Rightarrow $$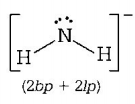
$$\left( {\text{D}} \right)PC{l_3} \Rightarrow $$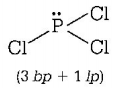
Thus, in $$PC{l_3},$$ the central $$P - {\text{atom}}$$ is surrounded by three bond pairs and one lone pair.
$$\left( {\text{A}} \right){H_2}O \Rightarrow $$

$$\left[ {bp = {\text{bond}}\,{\text{pair}}\,{\text{and}}\,lp = {\text{lone}}\,{\text{pair}}} \right]$$
$$\left( {\text{B}} \right)B{F_3} \Rightarrow $$

$$\left( {\text{C}} \right)NH_2^ - \Rightarrow $$

$$\left( {\text{D}} \right)PC{l_3} \Rightarrow $$

Thus, in $$PC{l_3},$$ the central $$P - {\text{atom}}$$ is surrounded by three bond pairs and one lone pair.