Question
Which of the following represents the given mode of hybridisation $$s{p^2} - s{p^2} - sp - sp$$ from left to right ?
A.
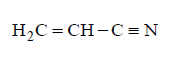
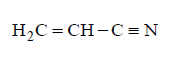
B.
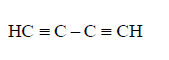
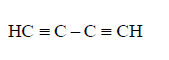
C.


D.
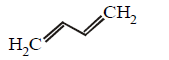

Answer :
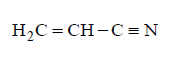
Solution :
$$\eqalign{ & \mathop {C{H_2}}\limits_1 = \mathop C\limits_2 H - \mathop C\limits_3 \equiv \mathop N\limits_4 \cr & 3\sigma \,{\text{bonds}}\,\left( {s{p^2}\,{\text{hybridisation}}} \right); \cr & 2\sigma \,{\text{bonds}}\left( {sp{\text{ - hybridisation}}} \right) \cr & {C_1} = 3\sigma \,{\text{bonds,}}{C_2} = 3\sigma \,{\text{bonds,}} \cr & {C_3} = 2\sigma \,{\text{bonds}} \cr} $$
$$\eqalign{ & \mathop {C{H_2}}\limits_1 = \mathop C\limits_2 H - \mathop C\limits_3 \equiv \mathop N\limits_4 \cr & 3\sigma \,{\text{bonds}}\,\left( {s{p^2}\,{\text{hybridisation}}} \right); \cr & 2\sigma \,{\text{bonds}}\left( {sp{\text{ - hybridisation}}} \right) \cr & {C_1} = 3\sigma \,{\text{bonds,}}{C_2} = 3\sigma \,{\text{bonds,}} \cr & {C_3} = 2\sigma \,{\text{bonds}} \cr} $$