Question
Which of the following molecules has the maximum dipole moment?
A.
$$C{O_2}$$
B.
$$C{H_4}$$
C.
$$N{H_3}$$
D.
$$N{F_3}$$
Answer :
$$N{F_3}$$
Solution :
$$C{O_2}$$ and $$C{H_4}$$ have zero dipole moment as these are symmetrical in nature. Between $$N{H_3}$$ and $$N{F_3}.$$ $$N{F_3}$$ has greater dipole moment though in $$N{H_3}$$ and $$N{F_3}$$ both, $$N$$ possesses one lone pair of electrons.
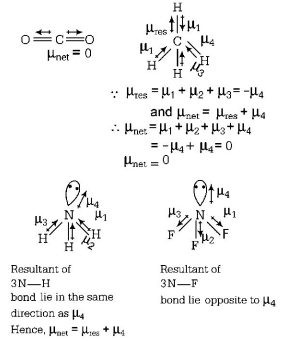
This is because in case of $$N{H_3},$$ the net $$N - H$$ bond dipole is in the same direction as the direction of dipole of lone pair but in case of $$N{F_3},$$ the direction of net bond dipole of three $$ - N - F$$ bonds is opposite than that of the dipole of the then lone pair.
$$C{O_2}$$ and $$C{H_4}$$ have zero dipole moment as these are symmetrical in nature. Between $$N{H_3}$$ and $$N{F_3}.$$ $$N{F_3}$$ has greater dipole moment though in $$N{H_3}$$ and $$N{F_3}$$ both, $$N$$ possesses one lone pair of electrons.

This is because in case of $$N{H_3},$$ the net $$N - H$$ bond dipole is in the same direction as the direction of dipole of lone pair but in case of $$N{F_3},$$ the direction of net bond dipole of three $$ - N - F$$ bonds is opposite than that of the dipole of the then lone pair.