Question
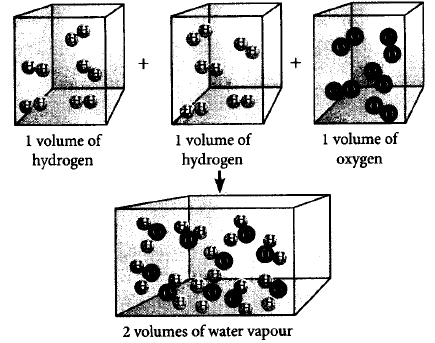
Which of the following laws of chemical combinations is satisfied by the figure ?
Which of the following laws of chemical combinations is satisfied by the figure ?
A.
Law of multiple proportion
B.
Law of multiple proportion
C.
Gay Lussac's law of gaseous volume
D.
Law of conservation of mass
Answer :
Gay Lussac's law of gaseous volume
Solution :
According to Gay Lussac's law of gaseous volume, when gases combine or are produced in a chemical reaction, they do so in simple ratio by volume provided all gases are at same temperature and pressure.
$$\mathop {2{H_2}}\limits_{2\,vol.} + \mathop {{O_2}}\limits_{1\,vol.} \to \mathop {2{H_2}O}\limits_{2\,vol.} $$
According to Gay Lussac's law of gaseous volume, when gases combine or are produced in a chemical reaction, they do so in simple ratio by volume provided all gases are at same temperature and pressure.
$$\mathop {2{H_2}}\limits_{2\,vol.} + \mathop {{O_2}}\limits_{1\,vol.} \to \mathop {2{H_2}O}\limits_{2\,vol.} $$