Question
Which of the following is the wrong statement?
A.
$$ONCl$$ and $$ON{O^ - }$$ are not isoelectronic.
B.
$${O_3}$$ molecule is bent
C.
Ozone is violet-black in solid state
D.
Ozone is diamagnetic gas.
Answer :
$$ONCl$$ and $$ON{O^ - }$$ are not isoelectronic.
Solution :
(A)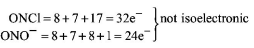
(B)
(C) Itisa pale blue gase. At $$ - {249.7^ \circ },$$ it forms violet black crystals.
(D) It is diamagnic in nature due to presence of paired electrons.
(A)
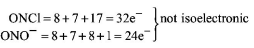
(B)

(C) Itisa pale blue gase. At $$ - {249.7^ \circ },$$ it forms violet black crystals.
(D) It is diamagnic in nature due to presence of paired electrons.