Question
Which of the following is the electron deficient molecule?
A.
$${B_2}{H_6}$$
B.
$${C_2}{H_6}$$
C.
$$P{H_3}$$
D.
$$Si{H_4}$$
Answer :
$${B_2}{H_6}$$
Solution :
$${B_2}{H_6}$$ is electron deficient molecule because boron atom has three half-filled orbitals in excited state. The structure of $${B_2}{H_6}$$ is represented as follows:
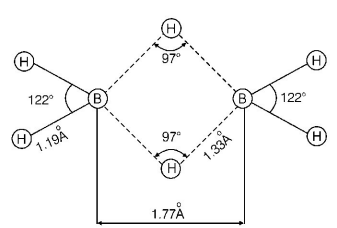
In it two electrons of a $$B— H$$ bond are involved in formation of three centre bond, these bonds are represented as dotted lines.
$${B_2}{H_6}$$ is electron deficient molecule because boron atom has three half-filled orbitals in excited state. The structure of $${B_2}{H_6}$$ is represented as follows:

In it two electrons of a $$B— H$$ bond are involved in formation of three centre bond, these bonds are represented as dotted lines.